6
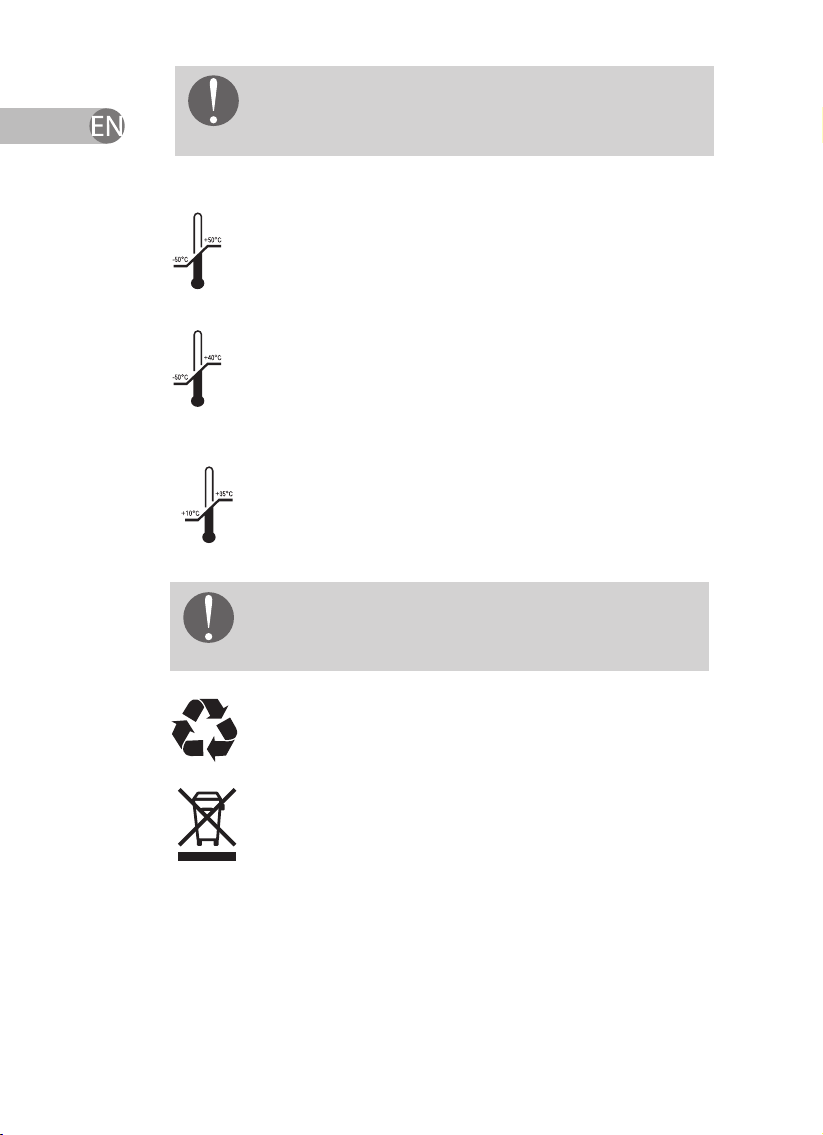
Operational conditions: temperature from +10
°C to +35 °C, relative air humidity from 30 to 93%,
atmospheric pressure from 70 to 106 kPa (525 to
795 mm Hg).
Attention! If the device has been stored at the
temperature below 10 °C, keep it in normal climate
conditions for no less than 2 hours - before use.
Utilization: All packaging materials are not
environmentally harmful, they may be used
repeatedly.
Separate collection of electrical and electronic
equipment.
Transportation conditions: temperature from -50
°C to +50 °C, relative air humidity from 30 to 93%,
atmospheric pressure from 70 to 106 kPa (525 to
795 mm Hg
Storage conditions: temperature from -50 °C
to +40 °C, relative air humidity from 30 to 93%,
atmospheric pressure from 70 to 106 kPa (525 to
795 mm Hg
Attention! If the patient is running through a course
of other physical therapy, use of the device is possible
only under approval of attending physician.
Product disposal is technically possible. The product
poses no danger to human life, health and the
environment after the end of its life (operation) and does
not require special measures for preparing and shipping
the compound parts of the product for recycling.