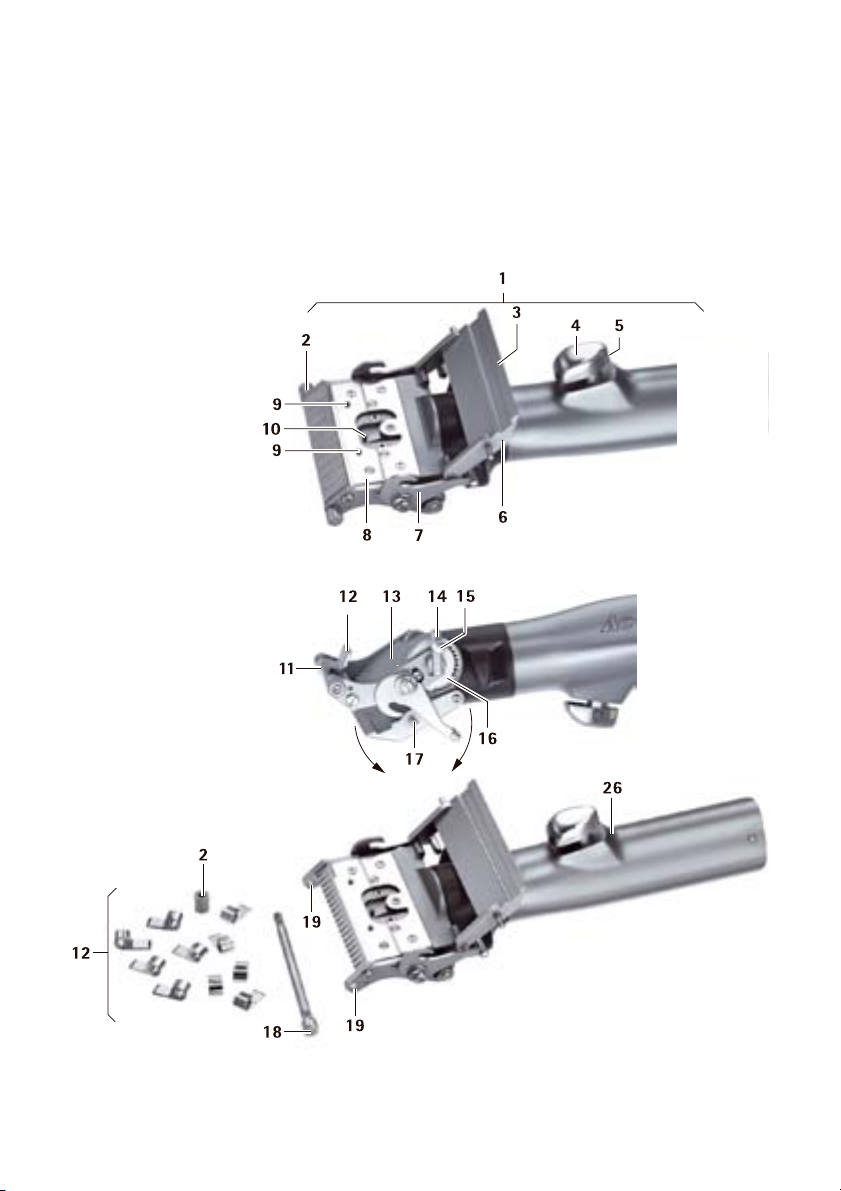
3
Contents
1. Safe handling and preparation ...........................4
2. Product description ................................................ 4
2.1 System components ............................................... 4
2.2 Components necessary for use ........................... 4
2.3 Intended use ............................................................. 5
2.4 Operating principle ................................................. 5
3. Preparation ............................................................... 5
4. Working with the Acculan® 3Ti Dermatome .. 5
4.1 System set-up .......................................................... 5
Installing the flap rod of
the Acculan® 3Ti Dermatome.............................. 5
Dismantling the flap rod of
the Acculan® 3Ti Dermatome.............................. 5
Connecting the accessories.................................. 6
Inserting the dermatome blade........................... 6
Removing the dermatome blade......................... 7
Inserting the battery.............................................. 7
Removing the battery ............................................ 9
Intraoperative battery change........................... 10
Protection against inadvertent activation..... 11
Intraoperative storage......................................... 11
4.2 Function checks .....................................................12
4.3 Safe operation .......................................................12
Adjusting the cutting depth of
the Acculan® 3Ti Dermatome............................ 12
Adjusting the cutting width of
the Acculan® 3Ti Dermatome............................ 12
Operating the Acculan® 3Ti Dermatome........ 13
Taking skin grafts.................................................. 13
5. Validated processing procedure .......................14
5.1 Single-use products .............................................14
5.2 General notes .........................................................14
5.3 Preparations at the place of use ......................15
5.4 Preparation prior to cleaning ............................15
5.5 Cleaning/Disinfecting ..........................................16
5.6 Manual cleaning/disinfecting ...........................17
Manual cleaning and wipe disinfection......... 17
5.7 Mechanical cleaning/disinfecting ....................18
Mechanical alkaline cleaning and
thermal disinfecting............................................. 19
5.8 Inspection, maintenance and checks ..............19
5.9 Packaging ................................................................19
5.10 Sterilization method and parameters .............20
5.11 Sterilization for the US market .........................20
5.12 Storage .....................................................................20
6. Maintenance ..........................................................21
7. Troubleshooting list .............................................22
8. Technical Service ..................................................23
9. Accessories/Spare parts ......................................23
10. Technical specifications ......................................24
10.1 Ambient conditions ..............................................24
11. Disposal ...................................................................25
12. Distributor in the US/Contact in Canada
for product information and complaints .......25
Direction of rotation to
tighten the nut
Service label on Acculan® 3Ti
Dermatome
Notice indicating the next
scheduled service (date) by an
international B. Braun/
Aesculap agency, see
Technical Service