DEHAS QualityMix N2O Series User manual

1
Contents
1. Explanation of the most important abbreviations......................................................... 2
2. Safety information - warnings, safety precautions and labelling information................ 2
3. Package contents and inspection upon receipt........................................................... 3
4. Intended application.................................................................................................... 4
5. Before first use............................................................................................................ 4
6. Technical Data............................................................................................................ 6
7. Pressure drop in the system ....................................................................................... 7
8. Transport and storage requirements........................................................................... 7
9. Dryness and composition of gas supplies................................................................... 7
10. Illustrations and naming of components...................................................................... 8
11. Installation .................................................................................................................10
12. Alarm test ..................................................................................................................10
13. Initial operation ..........................................................................................................11
14. Cleaning and disinfection...........................................................................................12
15. Maintenance..............................................................................................................12
16. Return of Goods ........................................................................................................13
17. Disposal.....................................................................................................................13
18. Troubleshooting.........................................................................................................14
19. Warranty conditions...................................................................................................15
Status: 11/2019

2
1. Explanation of the most important abbreviations
FIO2
Fractional concentration of inspiratory oxygen
DISS
Diameter Index Safety System
NIST
Non-interchangeable Screw Thread System
Bar
Unit of measurement for pressure
l/min
Litres per minute
2. Safety information - warnings, safety precautions and
labelling information
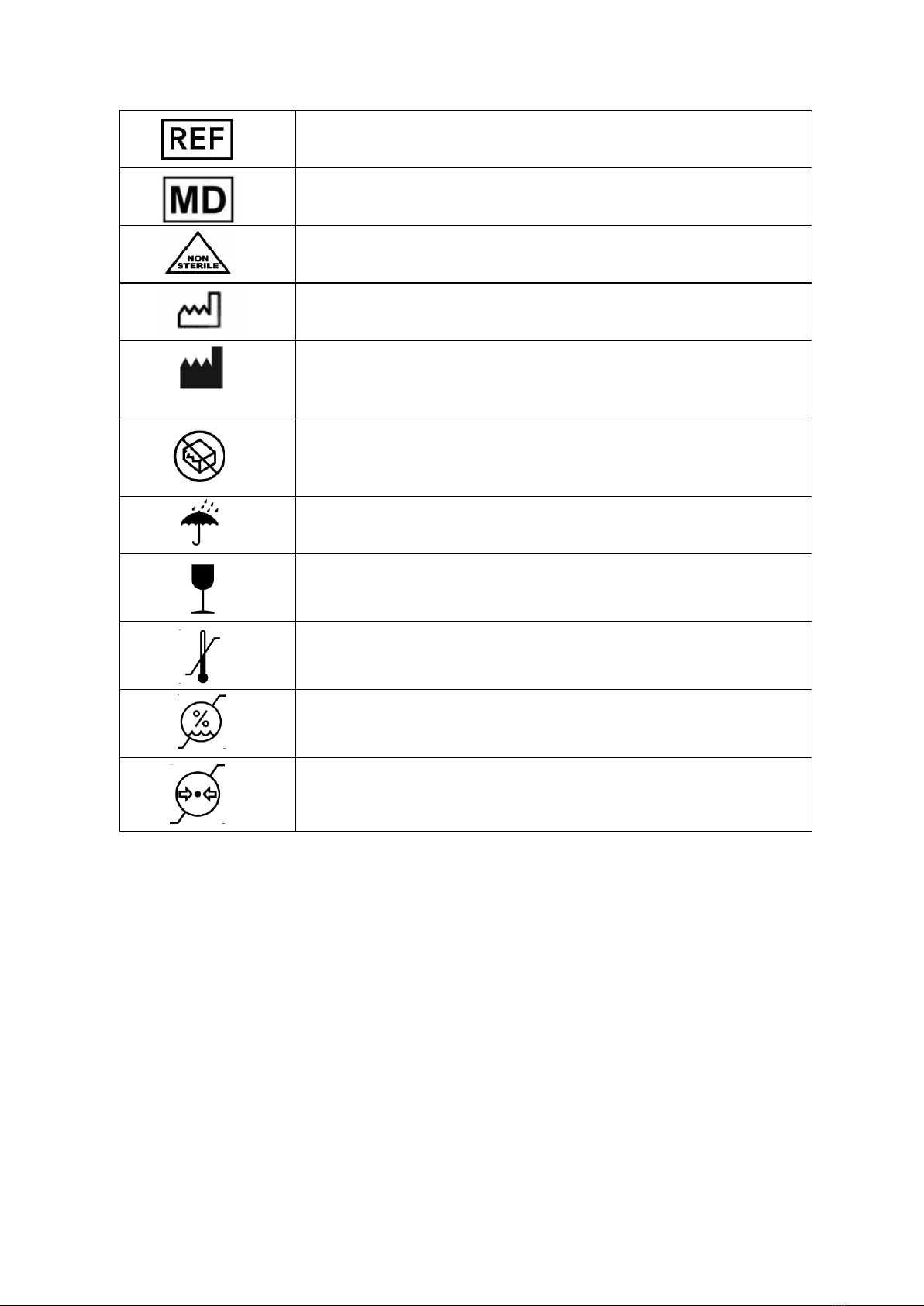
Symbol
Description
This symbol indicates that the device complies with the
requirements of Regulation 93/42/EEC regarding medical
devices and all applicable international standards.
WARNING
Indicates a potentially hazardous situation which, if not
avoided, could result in death or serious injury.
ATTENTION
Use of this symbol indicates a potentially hazardous
situation which, if not avoided, could result in equipment
damage.
or
Indicates the need for the user to consult the user
manual.
DO NOT USE OIL
Indicates the serial number of the manufacturer so that a
specific medical device can be identified.
3
Indicates the manufacturer's order number so that the
medical device can be identified.
Medical device
Non-sterile
Date of manufacture
Indicates the manufacturer of the medical device
according to EU Directives 90/385/EEC, 93/42/EEC and
98/79/EC.
Indicates a medical device that should not be used if the
packaging is damaged or opened.
Designates a medical device that must be protected
against moisture.
Designates a medical device that may break or be
damaged if handled carelessly.
Describes the temperature limits to which the medical
device can be safely exposed.
Indicates the humidity range to which the medical device
can be safely exposed.
Indicates the range of atmospheric pressure to which the
medical device can be safely exposed.
3. Package contents and inspection upon receipt
Package contents: 1 Basic unit consisting of:
11 mixer with adjustment unit
1Nitrous oxide module with flush function
2Connection hoses (O2 & N2O)
2 Pressure reducer 3.8 Bar (O2& N2O)
1User manual
According to order option
1Demand System N2O with connection hose

4
Or
1Flow meter with connection block and reservoir bag
Inspection: Remove the device from its packaging and inspect it for
damage. If you notice any damage, DO NOT use the device
and contact your distributor.
4. Intended application
The QualityMix N2O oxygen/nitrous oxide mixer is designed for the administration
of a continuous and accurate mixture of medical nitrous oxide and medical
oxygen to infants, children and adults via the exit port. The exact fractionated
inspiratory oxygen nitrous oxide concentration (FIO2/FIN2O) corresponds to the
selected FIO2/FIN2O setting on the control knob (rotary selector).
Indication:
This device is to be used by patients under the supervision of trained specialist
personnel for pain therapy.
Contraindication:
Do not use with patients who cannot breathe independently. Do not use for life
support or life saving.
5. Before first use
Read all instructions before use!
These instructions for use provide qualified personnel with instructions for
installing and operating the QualityMix N2O. The instructions are intended for
your safety and to protect the device from damage. If you do not understand any
information or instructions in this instruction manual, do not use the device and
contact your supplier.
DANGER
This product is not intended for use as a life-saving or life-supporting device.
WARNING
The QualityMix N2O oxygen/nitrous oxide mixer should only be operated
by medical personnel under the direct supervision of a licensed
physician
5
The QualityMix N2O oxygen/nitrous oxide mixer should be used only for
the purposes described in these operating instructions.
Review the prescribed dose before administration to the patient and
monitor the administration frequently
The QualityMix N2O oxygen/nitrous oxide mixer may only be serviced
by a qualified technician
Always comply with EN and DIN standards for the handling of medical
gas products, flow meters and oxygen
The oxygen/nitrous oxide concentration must be confirmed with an
oxygen analysis/monitoring device
DO NOT interfere with the alarm
DO NOT use the mixer when the alarm sounds
DO NOT use oil in or near the mixer
DO NOT use the mixer near flames, combustible/explosive materials,
vapours or gases
NEVER smoke in an area where oxygen is administered
When administering O2/N2O, personnel may be exposed to N2O. The
applicable national laws specifying exposure limits and safety
regulations for handling medical gases in the workplace must be
complied with. Possible exposure can be prevented by continuous,
effective control of the system, ventilation and working practices
The oxygen concentration rotary switch cannot be rotated 360 degrees.
Turning the switch to less than 30% or more than 100% oxygen will
damage the mixer
WARNING
Close the gas supply when the QualityMix N2O oxygen/ nitrous oxide
mixer is not being used
Always ensure an adequate supply of the gases used. After each use,
check the level of the gas source before reuse
Store the QualityMix N2O oxygen/nitrous oxide mixer in a clean, dry place
when not in use
The QualityMix N2O oxygen/nitrous oxide mixer does NOT contain
magnetic materials containing iron and is MRI compatible (max 3 Tesla). A
clearance of 2 meters must be maintained
The O2monitor is not suitable as an accessory for MRT
ATTENTION
Always ensure that all connections are secure and tight
NOT suitable for sterilization
DO NOT immerse in liquids
DO NOT sterilize with ethylene trioxide (EtO)
DO NOT use if dirt or impurities are present on or near the mixer or
connectors
6
DO NOT clean with aromatic hydrocarbons
The inlet pressure of the supply source must correspond with the
specifications of the mixer.
When using any gas source, always use a pressure reducer
within a pressure range of 3.2 to 3.8 bar
6. Technical Data
Model:
QualityMix N2O Variants:
QualityMix N2O 50 FIX
QualityMix N2O 50
QualityMix N2O 70 (Mechanical stop at
50% N2O)
Main output flow
1-120 l/min
Emergency flow (failure of
nitrous oxide or oxygen supply)
>50 l/min
FLUSH Button
100% O2 ca. 50 Litre/Minute
Alarm activation when supply
pressure drops
Alarm on - at a pressure difference between
the two gases of approx. 0.9 bar.
Alarm off - if the pressure difference between
the two gases is > 0.3 bar.
Example: Input pressure 3.8 bar.
Alarm on at 2.7-2.4 bar. Alarm off at a
maximum of 3.5 bar
Alarm volume
≥80 dB at a distance of 1 m.
Adjustment range of
N2O concentration
0 - 70 % N2O
With a mechanical barrier at 50% nitrous
oxide concentration.
Results in an N2O value equivalent to an O2
concentration of between 100% and 30%
Gas inlet pressure with
required pressure reducer
3.2 - 3.8 bar nitrous oxide and oxygen within
max. 0.7 bar pressure differential
7
Accuracy of the mixed gas
(FIO2)*
± 3 % oxygen
Connection types
1 x DISS output for mixed gas and 1 x NIST
input for nitrous oxide N2O and oxygen O2
respectively
Dimensions LxBxH
13 x 16.5 x 18.2 cm
Weight
2100 g
Operating temperature
+5°C to +50°C
The QualityMix N2O oxygen/nitrous oxide mixer has been degreased for oxygen
utilization prior to delivery. The reversed gas flow of the oxygen nitrous oxide
mixer complies with Clause 9 of ISO 11195:2018. The oxygen analyser used
must comply with ISO 80601-2-55 and CE regulations.
7. Pressure drop in the system
Low flow
≤0.14 bar with inlet pressures of 3.2-3.8 bar and a
flow rate of 10 l/min at >50% FIN2O
High flow
≤0.21 bar at inlet pressures of 3.2-3.8 bar and a
flow rate of 30 l/min at 50% FIN2O
8. Transport and storage requirements
Temperature range
-20 °C to 50 °C
Humidity
max. 95% non-condensing humidity
9. Dryness and composition of gas supplies
Nitrous oxide (N2O):
The medical nitrous oxide must meet all of the requirements for medical nitrous
oxide (N2O) according to the European Pharmacopoeia.
Oxygen (O2):
The oxygen used must meet all of the requirements for medical oxygen (O2)
according to the European Pharmacopoeia..

8
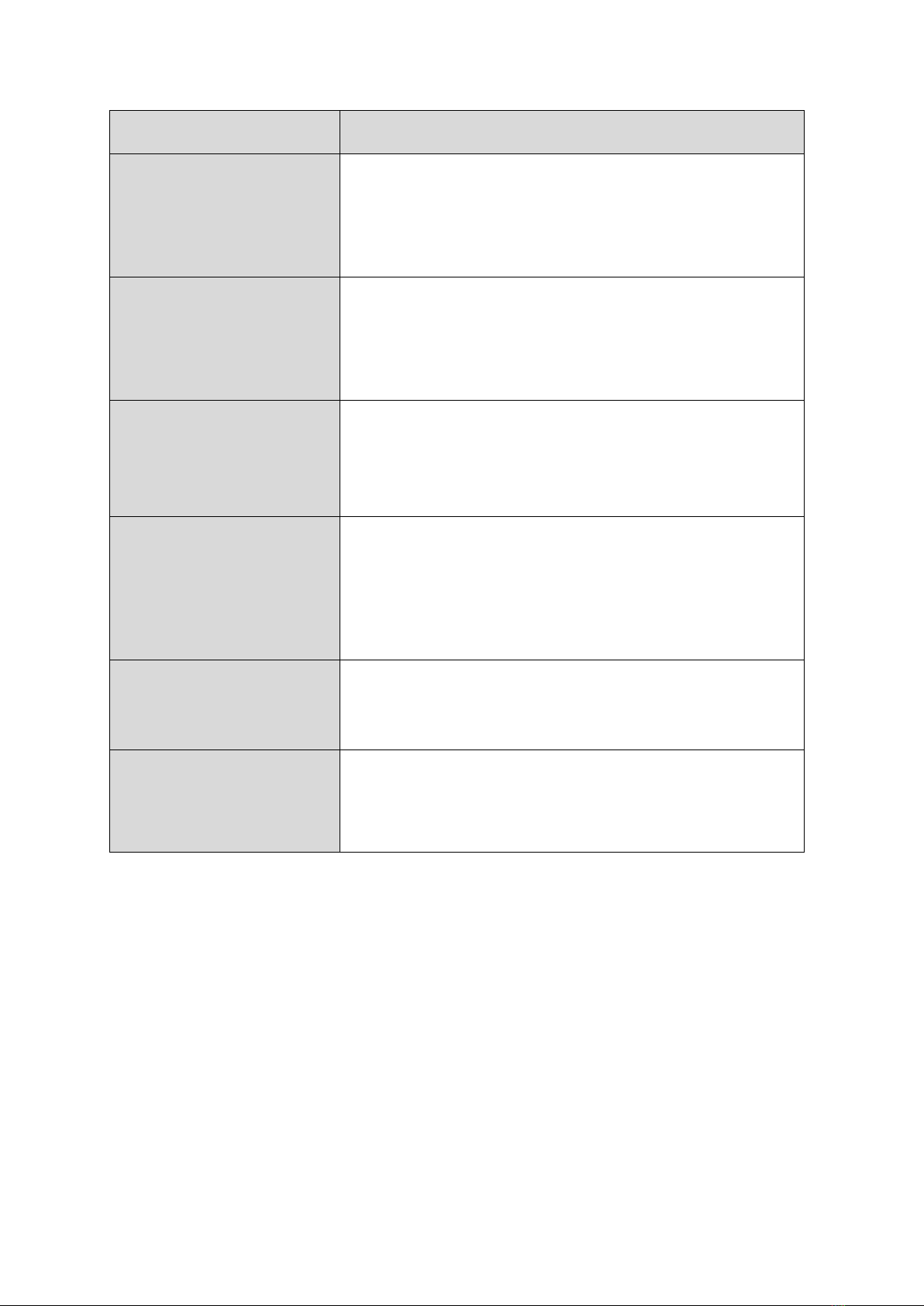
10. Illustrations and naming of components
ATTENTION
If you follow the instructions regarding processing, the labelling on the devices
will remain intact. If the labelling should nevertheless become illegible or
missing, please contact the manufacturer or your local contact person.
This illustration depicts the QualityMix N2O
Main output
DISS connection
For Flowmeter
or N2O demand
valve
NIST connector for
medical oxygen O2
Alarm
Flush O2
Button
NIST connector for
medical nitrous
oxide N2O
Rear - standard rail
bracket connection
Flowmeter
Rotary switch
concentration O2and
N2O with scaling
Supplementary
module N2O
Flowmeter
adjustment knob
(Flow)
9
Component
Description
Rotary switch for
oxygen and nitrous
oxide concentrations
A rotary switch for setting the oxygen
concentration to between 21 % - 100 %. The FIO2
scale is for reference purposes only. This rotary
switch cannot be rotated 360°. The rotary switch
starts at 21% and extends to 100%.
Main outlet
A male threaded DISS connector with shut-off
valve that provides gas flow when connected to a
control device such as a flow meter with reservoir
bag or an N2O demand system.
Connection for medical
oxygen
A NIST oxygen connection with female thread and
one-way valve for connecting an oxygen supply
hose.
Connection for medical
nitrous oxide
A male threaded NIST connector and a one-way
valve to connect a nitrous oxide supply hose.
Alarm
An audible alarm that sounds in the event of
excessive pressure drop or failure of oxygen and/or
nitrous oxide supply.
Flush O2button
For administration of 100% O2, independent of the
concentration setting on the rotary switch of the
QualityMix N2O mixer.

10
11. Installation
Warning
Read the user manual before installing or using the unit.
Monitor the oxygen and nitrous oxide concentration with an oxygen
analyzer/monitoring device.
Attention
Check the QualityMix N2O mixer for visible damage before use and do not use
if it is damaged.
Note: Perform the following test before operating the device for the first
time
Alarm test (see section 12)
Preparation for the alarm test
1. Attach the QualityMix N2O oxygen/nitrous oxide mixer to a rail or support
rod in an upright position.
2. Connect the nitrous oxide and oxygen supply lines to the appropriate
inlet ports at the bottom of the mixer.
3. Connect a flow meter or N2O demand valve to one of the output ports.
Flow capacity of the output:
N2O mixer, all variants: 0 –120 l/min
4. Connect an exhaust line to the outlet port of the flow meter or to the
intended connection of the N2O demand valve.
12. Alarm test
1. Connect the QualityMix N2O mixer to the nitrous oxide and oxygen
sources, pressurize the mixer and turn the flow meter anti-clockwise.
2. Set the rotary switch for nitrous oxide concentration to 50% (FIN2O).
3. Disconnect or switch off the nitrous oxide supply to the QualityMix N2O
oxygen/nitrous oxide mixer. The mixer should make a loud beeping
11
sound as an alarm. This sound indicates that the alarm is working
properly.
4. Reconnect and activate the nitrous oxide supply to the mixer; the
beeping should stop.
5. Disconnect or switch off the oxygen supply to the mixer. The beeping
sound indicates that the alarm is functioning properly.
6. Reconnect and activate the oxygen supply to the mixer; the beeping
sound should stop.
7. If the alarm does not function correctly, DO NOT USE the unit.
13. Initial operation
Attention
Before use, check the QualityMix N2O oxygen/nitrous oxide mixer for visible
damage and do not use if it is damaged.
1. Attach the mixer to the rail or stand bracket.
2. Connect the nitrous oxide and oxygen supply lines to the mixer and
supply.
3. Connect the flow meter or the N2O demand system to the output of the
mixer.
4. Set the nitrous oxide concentration rotary switch to the prescribed value.
5. Check the flow of the oxygen/nitrous oxide mixture to the patient.
6. Check the oxygen/nitrous oxide concentration with an oxygen
analysis/monitoring device.
7. When the oxygen/nitrous oxide mixer is not in use, shut off the gas supply
or disconnect the appliance from the gas supply.
12
14. Cleaning and disinfection
Attention
NOT suitable for sterilization.
NEVER immerse the QualityMix N2O oxygen nitrous oxide mixer in any
liquid
DO NOT use strong solvents or abrasives
DO NOT clean with aromatic hydrocarbons
The outside of the device must be disinfected at regular intervals or at
least after each patient in accordance with the applicable hygiene
standard.
1. Disconnect all gas connections and equipment before cleaning.
2. Wipe the outside with a cloth moistened with non-oxidizing disinfectant
and water.
3. Wipe dry with a dry cloth.
The manufacturer recommends the use of Dismozon plus® disinfectant,
manufactured by Bode Chemie GmbH & Co.
15. Maintenance
The following maintenance and inspection tasks must be carried out:
•Monthly check of the alarm by the user
•A safety inspection (SI) must be carried out every year by a trained
operator or medical technician.
•Maintenance should be carried out at least every 2 years by trained
specialist personnel. Inspection of the reversed gas flow is part of the
maintenance and is therefore carried out every 2 years.

13
Reverse gas flow test
1. Set the nitrous oxide concentration of the mixer to 50%.
2. Connect the nitrous oxide supply hose to the mixer and the gas supply and
open the supply.
Measure the flow rate at the oxygen inlet using a suitable measuring
instrument.
The flow rate should not exceed 10 ml/h.
If the flow rate is greater than 10 ml/h, the duckbill valve in the oxygen
inlet must be replaced in accordance with the service instructions and the
measurement repeated.
3. Connect the oxygen supply hose to the mixer and the gas supply, and
open the supply.
Measure the flow rate at the nitrous oxide inlet using a suitable measuring
instrument.
The flow rate should not exceed 10 ml/h.
If the flow rate is greater than 10 ml/h, the duckbill valve in the nitrous
oxide inlet must be replaced in accordance with the service instructions
and the measurement repeated.
16. Return of Goods
Please contact your distributor regarding this matter. They will coordinate the
return for you. It is important that you provide a description of the problem so
that the return can be processed in a targeted manner. All returns must be sent
in sealed containers to avoid damage. The distributor is not responsible for
equipment that is damaged during transport.
17. Disposal
This device and its packaging do not contain any hazardous substances. No
special precautions are required when disposing of the device and/or its
packaging.
Please recycle

14
18. Troubleshooting
If the oxygen/nitrous oxide mixer fails, refer to the troubleshooting section
below. If this does not solve the problem, please contact your local distributor.
Problem
Possible cause
Remedy
Discrepancy between
the setting of the
oxygen concentration
on the mixer and on
the
analysis/monitoring
device (more than 3
%)
1. Pre-pressure too
unequal/too low
Pre-pressure
inspection: optimum
pre-pressure 3.9 to 6.5
Bar
2. Analysis/monitoring
device does not
accurately register
Recalibrate the
monitoring device or
use a different
analysis/calibration
monitoring device
3. Gas supply
contaminated
Check gas supply with
calibrated oxygen
analyser/monitoring
device to ensure
oxygen content is
100% and nitrous
oxide content is 0%
4. The Flow of
downward mounted
device causes
backflow or
restricted flow
Disconnect the mixer.
Check the oxygen
concentration at the
mixer outlet
No flow at the mixer
output
1. Gas supply turned
off
Turn on gas supply
2. Gas supply not
connected
Connect gas supply
Problem
Possible cause
Remedy
Alarm sounds
Difference between
oxygen and nitrous
oxide inlet pressures
higher than prescribed
Correct pressure
difference until oxygen
and nitrous oxide
pressures meet
specifications

15
19. Warranty conditions
The distributor guarantees that the mixer is free from defects in workmanship
and/or materials for the following period of time:
One (1) year after delivery
The distributor will, with written notice and with evidence that the device has
been stored, installed, serviced and operated in accordance with instructions and
standard industry practices and that no modifications, substitutions or
modifications have been made to the product, will correct such defect by
appropriate repair or replacement at the distributor’s expense.
VERBAL STATEMENTS DO NOT CONSTITUTE A GUARANTEE.
The distributor is not authorized to provide oral guarantees about the product
described in this agreement, and such statements are not binding and are not
part of the purchase agreement. Therefore, this second statement is the final,
complete and exclusive representation of the terms of this agreement.
The current version of the General Terms and Conditions of the distributor and
German law shall apply.

16
0482
Declaration of Compliance
DEHAS Medizintechnik GmbH
Wesloer Straße 112, Gebäude M
23568 Lübeck
GERMANY
QualityMix N2O 50 FIX
QualityMix N2O 50
QualityMix N2O 70
And the relevant accessories
Classification:
IIb
Classification Criteria:
Clause 3.2 Rule 11 of Annex IX of the MDD
We hereby declare under our sole responsibility that the products mentioned above comply
with the provisions of the following directives and standards of the EC Council. All
documents are retained on the premises of the manufacturer and the notified body.
Guidelines:
General application guidelines: Medical Device Directive (MDD),
Council Directive 93/42/EEC of 14 June 1993 Annex II.3 on medical
devices of the European Parliament.
Applied standards:
DIN EN 1041
EN ISO 14971
DIN EN ISO 15001
DIN EN ISO 15002
DIN EN ISO 15223-1
DIN EN 62366-1
ISO 11195
ISO 18562-1
ISO 18562-2
ISO 18562-3
ISO 10993-1
Notified Body:
Medcert GmbH / 0482
Address:
Pilatuspool 2, 20355 Hamburg; GERMANY
Certificate number:
4153FR410180612 Expiry date: 11/2021
Already manufactured devices:
Valid from/to:
Traceability via serial number
06/ 2018 to expiry date
Manufacturer representative:
Jens Mittendorf
Position:
CEO /Head of Development
Date of issue:
27 November 2019

Your contact person for sales and service:
This manual suits for next models
3
Table of contents
Other DEHAS Measuring Instrument manuals