Supplement Zeus Infinity Empowered 3
English
Reprocessing
Safety information
Appropriate reprocessing
SpiroLife flow sensor
WARNING
Risk due to inappropriately reprocessed prod-
ucts
Reusable products must be reprocessed, oth-
erwise there is an increased risk of infection.
– Follow the infection prevention policies
and reprocessing regulations of the
health-care facility.
– Follow the national infection prevention
policies and reprocessing regulations.
– Use validated procedures for reprocess-
ing.
– Reprocess reusable products after every
use.
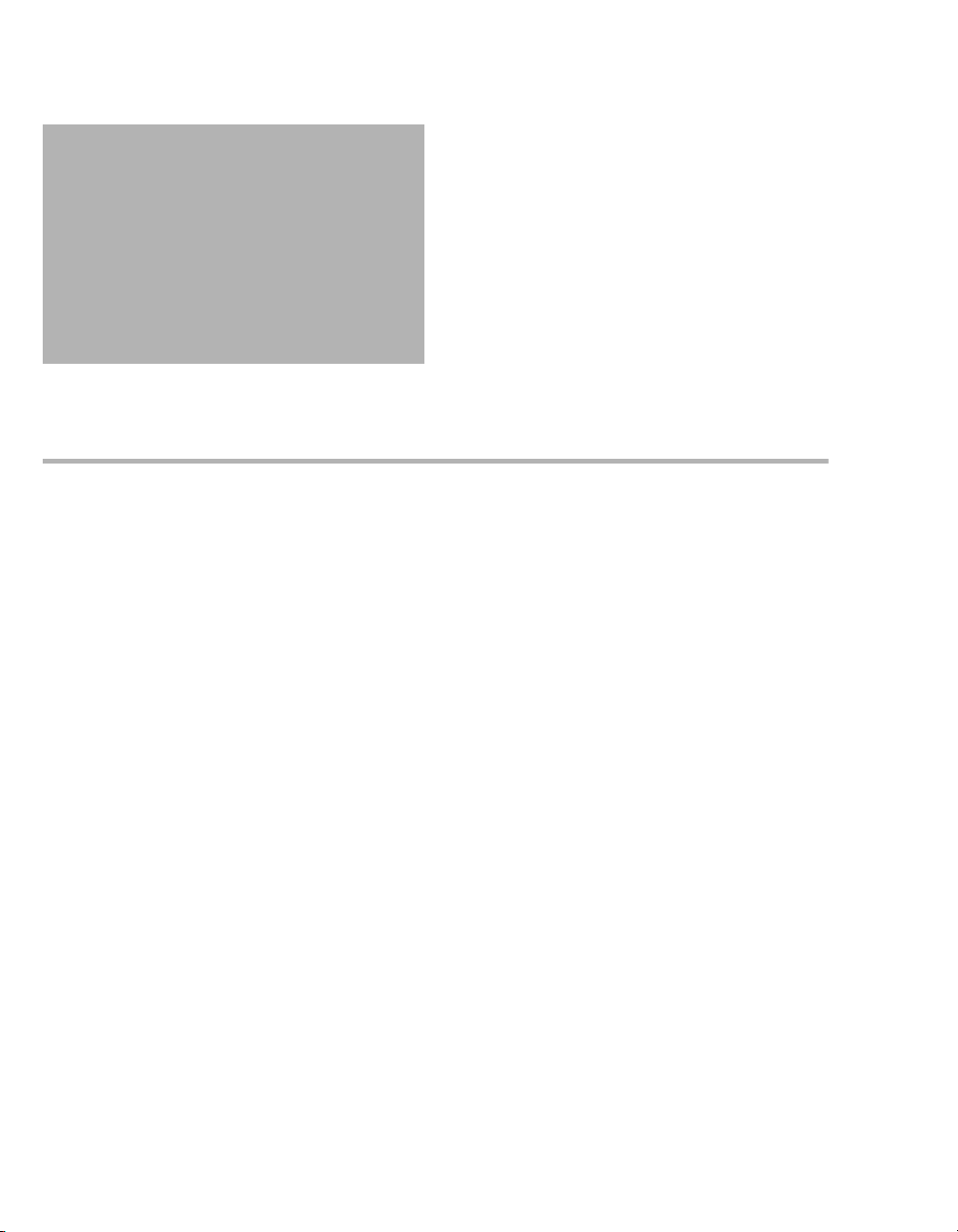
– Follow the manufacturer's instructions for
cleaning agents, disinfectants, and repro-
cessing devices.
WARNING
Risk due to faulty products
Signs of wear, e.g., cracks, deformation, dis-
coloration, or peeling, may occur with repro-
cessed products.
Check the products for signs of wear. Replace
them if necessary.
CAUTION
Risk of failure of flow measurement
Improper reprocessing and soiling, such as de-
posits or particles, may damage the flow sensor:
– No machine cleaning or disinfection of the
sensor insert
– No plasma sterilization or radiation steriliza-
tion
– No water jets, compressed air, brushes, or
similar to be used on the sensor insert
– No ultrasonic bath
– Clean and disinfect the flow sensor in accor-
dance with the corresponding instructions for
use.
– For disinfecting the flow sensor use only clean
disinfectant solutions.
WARNING
Risk of fire
Residual vapors of highly flammable disinfec-
tants (e.g., alcohols) and deposits that were
not removed during reprocessing may ignite
when the flow sensor is in use.
– Ensure particle-free cleaning and disinfec-
tion.
– After disinfection, allow the flow sensor to
air for at least 30 minutes.
– Before inserting the flow sensor check for
visible damage and soiling, such as resid-
ual mucus, medication aerosols, and parti-
cles.
– Replace flow sensors when damaged,
soiled, or not particlefree.