EKF Quo-Test User manual

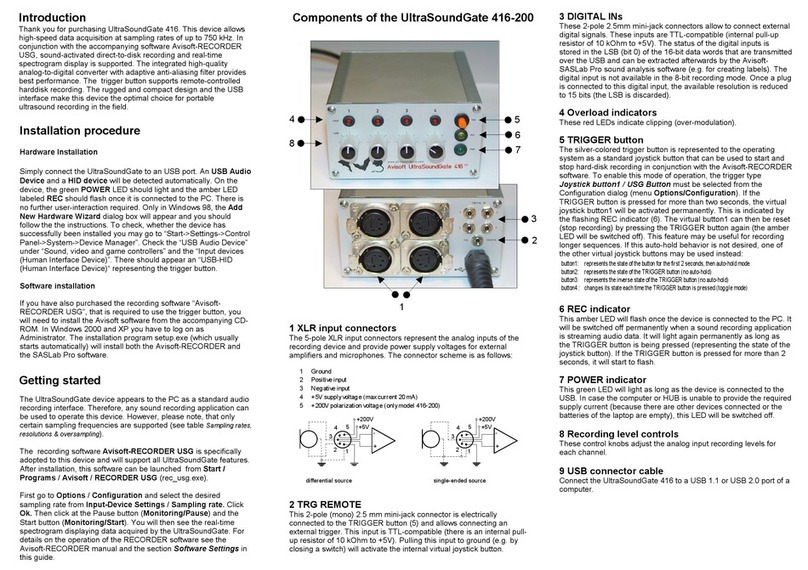
User Manual
Quo-Test
Diagnostics
for life

3121-9001-0167_EN.03.00_QT_Manual 04/2016
Copyright© 2016 EKF-diagnostic GmbH
No part of this publication may be
reproduced, transmitted, transcribed, stored
in any retrieval system or translated into any
human or computer language by any means
or in any form, without the prior written
permission of EKF-diagnostic GmbH.
Part Number: 3121-9001-0167
Quo-Test User Manual
Date: 04 / 2016
Issue: EN.03.00
For use with Firmware version 2.xx
Diagnostics
for life
EKF-diagnostic GmbH
Ebendorfer Chaussee 3
39179 Barleben
Germany
T +49 39203 511 0
www.ekfdiagnostics.com

3121-9001-0167_EN.03.00_QT_Manual 04/2016
Section 1 Safety Precautions
Section 2 Introduction
General Information
Unpacking
Warnings and Precautions
Intended Use
Quo-Test System Description
Measurement Principle
Section 3 Setting up the System
Setting the Language
Setting the Time
Setting the Date
Setting the Identification (ID) Options
Calibration
Reported Units
Functional Checking
Section 4 Running a Test
Preparing to Run a Test
Running the Test
Collecting the Sample
Scanning Operator and Patient ID
The Test Result
Printing the Test Result
Retrieving a Result from the Analyzer Memory
Running a Quality Control Sample
Section 5 Maintenance
Section 6 Troubleshooting Guide and Error Messages
Operational Error Messages
Section 7 Customer Support
Warranty
Disposal of the Analyzer
Returning an Analyzer
Section 8 Technical Specifications
Quo-Test Analyzer
Accessories
Section 9 Packaging Symbols and their Meaning
Contents

Section 1 - Page 1 of 1 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Basic safety precautions must always be taken, including all those listed below.
Danger - Misuse of electrical equipment can cause electrocution, burns,
re and other hazards.
Close supervision is necessary when the system is used, on, or near children,
or vulnerable persons.
Read the following instructions before using the system
1) Connect the analyzer to the mains supply. The mains outlet must have an earth
connection.
2) If the system is not going to be used for an extended period of time, unplug
the analyzer from the mains supply.
3) Do not place the system in liquid, or put it where it could fall into liquid.
If the equipment becomes wet, unplug from the mains supply before touching it.
4) Use the system only for the purpose described in this User Manual.
5) Do not use accessories which are not supplied or recommended by the
manufacturer.
6) Do not use the system if it is not working properly or if it has suffered any
damage, for example;
a) damage to the exible supply cord or its plug.
b) damage caused by dropping the system.
c) damage caused by dropping the system into liquid or splashing liquid on to it.
7) Do not let the equipment or the exible cords come into contact with surfaces
which are too hot to touch.
8) Do not block air openings or place the system on a soft surface. Keep air
openings free from debris.
9) Do not place anything on top of the system.
10) Do not drop or put anything into any opening in the equipment, unless
instructed by this User Manual.
11) Do not use the system where aerosol sprays are used, or where oxygen is
administered.
12) Do not use the system out of doors.
13) Do not use the system in close proximity to sources of strong electromagnetic
radiation, as these may interfere with the proper operation of the analyzer. An
evaluation of the electromagnetic environment should be undertaken before
using the system.
Section 1 Safety Precautions

Section 2 - Page 1 of 3
3121-9001-0167_EN.03.00_QT_Manual 04/2016
General Information
Please read these instructions in full before performing a test
The Quo-Test Analyzer is for in vitro diagnostic use only.
The Quo-Test Analyzer is for use at the point of care.
Only Quo-Test Cartridges can be used with the Quo-Test Analyzer.
Unpacking
Unpack the system and check that the following components are present in the pack.
If there is anything missing contact your local distributor or customer support.
• Quo-TestAnalyzer
• BarcodeScanner
• MainsPowerCable
• PowerSupply
• UserManual
It is recommended to keep the packaging to transport your analyzer. If the optional
printer has been purchased, it will be delivered in a separate box.
Warnings and Precautions
• Therearenouserserviceablepartsinsidetheanalyzer.Callyourlocaldistributoror
customer support if you have any problems that are not resolved by following the
Troubleshooting Guide and Error Messages section of this User Manual.
• Placethesystemonaclean,dry,atandlevelsurfaceawayfromdirectsunlight
in a room with a temperature range of 18 to 30 °C (64 to 86 °F). An analyzer which
has been stored at a temperature above or below the working temperature will take
longer to exit the Analyzer Warm Up screen when the system is rst powered on.
• Placethesystemawayfromdraughts,orothersourcesthatmaycausesudden
changes in temperature e.g. air ow from air conditioning units.
• Thesystemmustbewithineasyreachofamainssupplyoutletwithaprotective
earth connection.
• ThesystemhasbeendesignedandtestedtoCISPR11ClassA.Inadomestic
environment it may cause radio interference, in which case you may need to take
measures to mitigate the interference.
• Ensurethattheanalyzerandcartridgeshavereachedroomtemperaturebefore
using the system.
• TheanalyzermustbeusedinaccordancewiththeinstructionsstatedinthisUser
Manual.
• Usedcartridgesmustbetreatedanddisposedofasclinicalwaste.
Section 2 Introduction
IVD

Section 2 - Page 2 of 3 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Intended Use
TheQuo-TestA1CSystemisintendedforthein vitro quantitative determination of
glycated hemoglobin (HbA1c) in whole blood obtained from a nger prick or venous
whole blood sample collected into EDTA tubes.
TheQuo-TestA1CSystemisindicatedinthemanagementandtreatmentofdiabetes
mellitus and for monitoring long term glycemic control in patients diagnosed with
diabetes.
TheQuo-TestA1CSystemisdesignedforprofessionaluseonly.
Quo-Test Quality Controls are intended to check the correct operation of the Quo-Test
A1CSystem.
Quo-Test System Description
TheQuo-TestA1CSystemconsistsoftheQuo-TestAnalyzer,Quo-TestA1CTest
Cartridges,Quo-TestA1CControlKitandThermalLabelPrinter(optional).
Quo-Test A1C Test Cartridges can only be run on the Quo-Test Analyzer.
Figure 1 shows the main parts of the analyzer.
Figure 1: The Quo-Test Analyzer
Door
Rear Connection Panel
(see Figure 2 in Section 3 - Setting up the System)
Test Chamber
Key Pad
Display Screen
Slide

Section 2 - Page 3 of 3
3121-9001-0167_EN.03.00_QT_Manual 04/2016
ReadtheInstructionsForUsethatcomewiththeTestCartridges.Refertothe
Instructions For Use on how to prepare and perform the test. The Quo-Test Analyzer
will provide on screen instructions at each stage of performing the test. At the end
of the test the result is displayed on the screen, stored in the analyzer memory and
can be printed on to a label with the optional printer.
The Quo-Test Analyzer is calibrated by the manufacturer and there are no user-
serviceable parts.
The analyzer performs optical, electronic and mechanical checks on the test
cartridge throughout the test procedure and if a problem is detected the analyzer
stops the test and an error message is displayed on the screen. If this happens,
then consult the Troubleshooting Guide and Error Messages section of this User
Manual.
Measurement Principle
The arrangement of the two light sources and associated detectors allows the
Quo-Test Analyzer to be used as a dual channel spectrophotometer / uorimeter.
This allows the Quo-Test Analyzer to be used either to measure changes in optical
density as used in Immunoturbidimetric assays or changes in light transmission from
specic uoriphores as they interact with analytes of interest e.g. HbA1c.

Section 3 - Page 1 of 6 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Section 3 Setting up the System
Quo-Test
11:32 21/06/13
Setup File
Connect the barcode scanner and the printer (optional) to the analyzer by pushing
the cables into the connectors on the rear panel. Make sure the printer and barcode
scanner are connected to the correct ports as shown in Figure 2 below. If the printer
is no longer required, disconnect it from the analyzer.
Figure 2: Connection panel on the rear of the analyzer.
Connect the power supply to the analyzer and plug the supply cord into the
mains supply (100-240v AC 50-60Hz). Ensure that the supply has a protective
earth connection.
Once the analyzer has warmed up
the screen will show the Quo-Test
logo, this is the home screen. The
keypad under the blue screen has
three buttons. The text on the screen
immediately above each button shows
the function of that button. If there is
no text above a button, then it has no
function. The functions of the buttons
change, depending on which screen is
displayed.
USB Port
Model No.
Manufacturer
Serial No.
Barcode
Scanner Port
Printer
Port
DC
Power Supply

Section 3 - Page 2 of 6
3121-9001-0167_EN.03.00_QT_Manual 04/2016
When the analyzer is rst switched on, you must set the local language, time, date,
ID options and reported units before you perform a test. You only need to do this
once.
The analyzer should be switched off at the mains supply or unplugged when not
required for long periods. Otherwise the analyzer should be left on, but must be
powered down and restarted once a week, to perform the self-check procedure.
Ensure that the analyzer is displaying the home screen before powering the system
down.
PressthebuttonlabelledSetuptostartthesetupprocess.
Setting the Language
The default language for the analyzer
is English. To change to a different
language, press the Change button
and use the up/down arrow buttons to
select the required language from the
list.PresstheOK button to conrm the
selection.
Setting the Time
The analyzer has a clock that keeps track
of the date and time. Whenever a test is
performed, the result is recorded in the
analyzer memory with the date and time
that it was run. Choose Change on the
time screen to set the time.
Choose either the 24 hr or 12 hr clock
bypressingoneofthebuttons.Setthe
hours and minutes by using the up/
down arrowbuttons.PressOK when
correct.
If the12 hr clock is selected, then set
eitheramorpm.PressOK to conrm
the settings.
The time will then be displayed. Either
press the OK button to move on to
setting the date, see next section, or
press the Change button to go back
to setting the time.
Time
11:32
24 hr Change
Hour
11:32
OK pmam
Language
English
OK Change

Section 3 - Page 3 of 6 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Setting the Date
To set the date, use the up/down
arrow and OK buttons to set the year,
monthanddayasrequired.Pressthe
OK button to save the selection.
Choose the date format, either dd/mm/
yy or mm/dd/yy.PresstheOK button
to save the selection. The date will then
be displayed.
Either press the OK button to move on to setting the ID options, see next section, or
press Change to go back to setting the date.
Note: The analyzer will automatically adjust the time and date for leap years.
Setting the Identification (ID) Options
The Quo-Test Analyzer allows for operator and patient identication (ID) to be entered
via the supplied barcode scanner. This feature allows recording of the ID of the person
who ran the test and the person who was tested in the analyzer memory and on the
result print-out (if the optional printer is attached). These features can be switched on
or off. The operator has the option to skip the ID entry when analysing a cartridge if
the feature is turned on. Having the operator and patient ID stored or printed with the
test result, will aid identication of the result at a later date.
Barcode lengths up to 18 characters in Code 128, Code 39, NW7/Codabar and 2
of 5 Interleaved formats are supported. It is strongly recommend that users do not
use 2 of 5 interleaved as this format carries a risk of character substitution. Attempting
to scan a barcode in excess of 18 characters will result in the code being shortened
to 18 digits.
Users must verify that the Patient or
Operator ID scanned matches the one
displayed on the Quo-Test.
PressthePatient and Operator buttons
toswitchthesefeatureson.Pressthe
buttons again to switch the features off.
Finally, press OK to move on to setting
the reported units.
Year
2013
OK
Patient ID ON
Operator ID ON
OK Operator
Patient

Section 3 - Page 4 of 6
3121-9001-0167_EN.03.00_QT_Manual 04/2016
Calibration
TheQuo-TestA1CSystemiscertiedbytheNGSPandIFCC.
The Quo-Test Analyzer and A1C Test Cartridges have been calibrated using samples
providedbytheEuropeanReferenceLaboratoryviatheNGSPnetwork.
ResultsobtainedusingtheQuo-TestA1CSystemaretraceabletotheIFCCreference
method.
Reported Units
The Quo-Test A1C assay reports results in up to two user selectable units: % DCCT,
mmol/molIFCC,%JDS,eAGmg/dloreAGmmol/l.
mmol/mol IFCC = (% DCCT - 2.15) x 10.929
%JDS=(0.09274xmmol/molIFCC)+1.724
eAG mg/dl = (28.7 x % DCCT) – 46.7
eAG mmol/l = (1.59 x % DCCT) – 2.59
eAG values are based on a correlation study linking % DCCT to the patient’s average
glucose concentration, resulting in published formula to derive the eAG.
eAG values may differ signicantly from a patients glucose level measured at the
same time.
The Quo-Test Analyzer allows the user to select dual reporting (two different
measument units can be displayed) or single reporting. Users should refer to national
guidance when setting up reported units.
% DCCT is the default primary unit, to change the primary reported unit press Change
Primary Units
DCCT
OK Change

Section 3 - Page 5 of 6 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Secondary Units
IFCC
OK Change
Secondary Units
DCCT moreIFCC
Secondary Units
JDS moreNone
The rst screen allows you to select
DCCT (%), IFCC (mmol/mol) or select
More to see additional units.
If you select More, JDS (%) or More
are the options. If you select More you
are returned to the rst menu screen.
OnceaPrimaryreportableunitis
selectedyoucanselectaSecondary
Reportableunitto‘DualReport’.
IFCC (mmol/mol) is the default
secondary reportable unit. To select an
alternative secondary unit or switch off
dual reporting select Change.
The rst screen allows you to select
DCCT (%), IFCC (mmol/mol) or select
More to see additional units.
If you select More,JDS (%), None or
Morearetheoptions.SelectingNone
switchesoffdualreporting.Selecting
More will forward you to the eAG
options.
Primary Units
DCCT MoreIFCC
Primary Units
JDS More

Section 3 - Page 6 of 6
3121-9001-0167_EN.03.00_QT_Manual 04/2016
eAG can be reported in either mmol/l or
mg/dl to match the units used to report
glucose measurments locally.
Selecting More will return you to the rst
secondary units screen.
Once a secondary unit (or none) has
been selected, then setup is complete
and the analyzer is ready to run a test.
Note: Changing the secondary reported unit will also change the secondary reported
units on previously run samples held in the memory of the analyzer, as these are
calculated each time they are displayed. Changing the primary reported unit will not
alter the primary units reported on previously run samples.
Secondary Units
(mmol/l)
eAG
More(mg/dl)
eAG
Functional Checking
Each time the Quo-Test Analyzer is switched on it performs a series of functional
checks of the system and optics. The analyzer must be powered off and powered on
at least once a week to allow the analyzer to perform the internal self-checks.
RefertotheTroubleshooting Guide and Error Messages section of this User Manual
to diagnose any problems, if they occur.
Correct operation of the system may be conrmed by running Quo-Test A1C Control
samples.
To run a control, read the Instructions For Use supplied with your Quo-Test A1C
Control Kit in combination with the Running a Quality Control section of this User
Manual.

Section 4 - Page 1 of 8 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Preparing to Run a Test
Step 1
When the Quo-Test Analyzer
displays the home screen
with ‘Quo-Test’, the time and
date, the analyzer is ready
to run a test.
Step 2
Before using a new lot of test cartridges,
scan the calibration barcode that is
printed on the inner ap of the carton,
indicated by the symbol.
Placethebarcodescanneroverthe
calibration barcode and press the
barcode scanner button until you hear
a beep. The analyzer will conrm that
the data has been accepted, along with
displaying the lot number and expiry
date.
Section 4 Running a Test
2
1
Please read the Instructions For Use supplied with your Test
Cartridges.
Always use protective gloves when handling blood samples.
CAL

Section 4 - Page 2 of 8
3121-9001-0167_EN.03.00_QT_Manual 04/2016
Step 3
Removeapouchedcartridgefromthe
carton and place it next to the analyzer.
Allow at least 40 minutes for it to
equilibrate to the analyzer’s environmental
temperature before use, even if previously
stored at room temperature. When ready
to perfom the analysis, carefully open the
pouch and remove the Test Cartridge.
Check the indicator color of the silica gel
(see test Instructions For Use).
Do not handle the lower part of the
carrtridge containing the liquid.
Do not use the cartridge if condensation
is present.
Collecting the Sample
Step 4
The Quo-Test can be used with either
venous whole blood collected with EDTA
or with a nger prick blood sample.
For fringer prick sample collection, the
patient’s nger must be warm, dry and
clean, including being free of substances
such as hand cream. Use a single use
lancet (not provided) on the patient’s
nger to get a droplet of blood about the
same width as the Blood Collector.
For venous samples previously collected
into EDTA tubes, ensure that the sample
is thoroughly mixed and use standard
equipment to place a droplet of blood,
approximately 10 to 20 µl, onto a non-
metallic and non-absorbant surface such
asParalm®M.
Do not attempt to sample directly from
the tube.
Note:Paralm®M is a trademark of
PechineyPlasticsPackaging.
3
4

Section 4 - Page 3 of 8 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Step 5
Lightly touch the pointed tip of the
Blood collector to the top surface of the
droplet of blood, as per the image.
The blood will be taken up by the
collector.
The blood must completely ll the slot.
Note: avoid taking up air bubbles and
excess sample on the outside of the tip
of the Blood collector.
Do not wipe excess blood from the
collector.
Step 6
place the Blood collector into the free
cavity on the top of the Test cartridge,
making sure that the Blood collector is
ush with the top of the Test cartridge
and is not sticking out.
Note: Do not push the Blood collector
into the Test cartridge, as this may
cause an error message to be displayed
instead of a result. Refer to the
Troubleshooting Guide and Error
Messages section of this User manual
for further details.
Running a Test
Step 7
After the Test cartridge is prepared,
open the door of the analyzer and place
the cartridge into the test chamber,
making sure that the Test cartridge is
rmly seated. The Test cartridge must
be inserted into the analyzer within
1 minute of the blood sample being
collected.
5X
6
7

Section 4 - Page 4 of 8
3121-9001-0167_EN.03.00_QT_Manual 04/2016
Step 8
Pulltheredslidetowardsthefrontof
the analyzer and close the door. The test
will start automatically.
You will be prompted to scan the
OperatorandPatientID,ifthese
features have been activated.
Note:Iftheanalyzerdisplays“Scan
Lot ****** Data Card” then scan the
calibrationbarcode(seeStep2).
Step 9
When the test has nished the result will
be displayed on the screen and printed
on the (optional) printer, if connected.
Open the door and push the red slide
towardstherearoftheanalyzer.Remove
the used cartridge and dispose of it as
clinical waste.
Upon closing the analyzer door, the
display will return to the home screen
and the analyzer will be ready to run
another sample.
Note:SeeSection4-Retrieving a
Result from the Analyzer Memory 9
8

Section 4 - Page 5 of 8 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Scanning Operator and
Patient ID
IftheOperatorand/orPatientIDfeatures
are enabled (see section 3), when the
analyzer door is closed to start the test the
screen will request that the user scans the
Operatorand/orPatientID,depending
upon which features have been activiated
in the analyzer setup.
Pleasenotethatwhenusingthesystemin
QC mode the system will only request the
operator ID to be scanned, as the system
automaticallyusesthePatientIDforthe
Control Lot number.
Whenrequestedtoscanthe‘ID’,place
the barcode scanner over the Operator or
PatientIDbarcodeandpressthebarcode
scanner button.
When the analyzer has recorded the barcocde a beep will sound and the ID is
displayed on the screen. Conrm the displayed ID matches the barcode scanned. If
youdonothaveanOperatororPatientIDbarcodelabelavailable,presstheSkip
button to exit the screen. The test result will not be displayed until the requested ID’s
have been scanned or the screens have been exited. If an error occurs, the analyzer
willaborttherequesttoscantheOperatorand/orPatientIDifnotalreadycompleted.
Scan Operator ID
Skip
Scan Patient ID
Skip

Section 4 - Page 6 of 8
3121-9001-0167_EN.03.00_QT_Manual 04/2016
7.1% A1C
DCCT
54 mmol/mol IFCC
The Test Result
When the test is nished, the result will be displayed on the screen in the specied
units(Section3-Reported Units). The image below shows an example of the result
of a Quo-Test A1C Test. In this case, the test result was 7.1 % A1C. The letters “DCCT”
below the test result show the selected primary reported units of the test at the time
it was run. Important: make a note of the calibration scheme in order to accurately
compare results. Below the main result is a derived result showing the equivalent value
in mmol/mol IFCC units.
The result will stay on the screen until
the analyzer door is opened. The result
is stored in the analyzer memory (see
Section4-Retrieving a Result from the
Analyzer Memory). On removal of the
used cartridge, the analyzer will be ready
to perform another test.
Printing the Test Result
If you have the optional printer
connected, the result will be printed
when the test has nished. The result is
printed onto a self-adhesive label to be
kept with the patients’ records.
Time:
Date:
Result:
Lot:
Inst ID:
Test ID:
Patient:
Operator:
Quo-Test A1C
11:34
21/06/13
7.1 % A1C DCCT
54 mmol/mol IFCC
020130
021132
00053
xxxxxxxxxxxxxxxx
xxxxxxxxxxxxxxxx

Section 4 - Page 7 of 8 3121-9001-0167_EN.03.00_QT_Manual 04/2016
Retrieving a Result from the Analyzer Memory
The results of the most recent 7,000 tests run on the analyzer are recorded in the
memory.Onlythemostrecent1,000testresultscanbeviewedontheanalyzer.Press
the File button on Quo-Test home screen. The most recently performed test result
will be shown on the screen.
The sequential number of the test (in the
example below it is Test 53) will be shown
together with the time and date the test
was started along with the result. If the
OK button is pressed a second page will
be shown which includes the Operator
andPatientIDsandthecartridgelot
number.
PressthePrint button for a printed
copy of the result, if the optional printer
is attached, or press the Exit button to
returntothehomescreen.PresstheBack
button to return to the memory le.
The most recent 1,000 test results may
be accessed using the up/down arrow
buttons on the result screen as shown
above. To nd earlier test results contact
your local distributor or customer support.
Note: Changingthesecondaryreportedunit(seeSection3-Report Units) will also
change the secondary reported units on previously run samples held in the memory
of the analyzer, as these are calculated each time they are displayed. Changing
the primary reported unit will not alter the primary units reported on previously run
samples.
Test 00053
11.34 pm 21/06/13
7.1% A1C DCCT
54 mmol/mol IFCC
OK
Test 00053
Lot: 020130
Pat ID:
Op ID:
Print Exit Back
Table of contents
Other EKF Measuring Instrument manuals