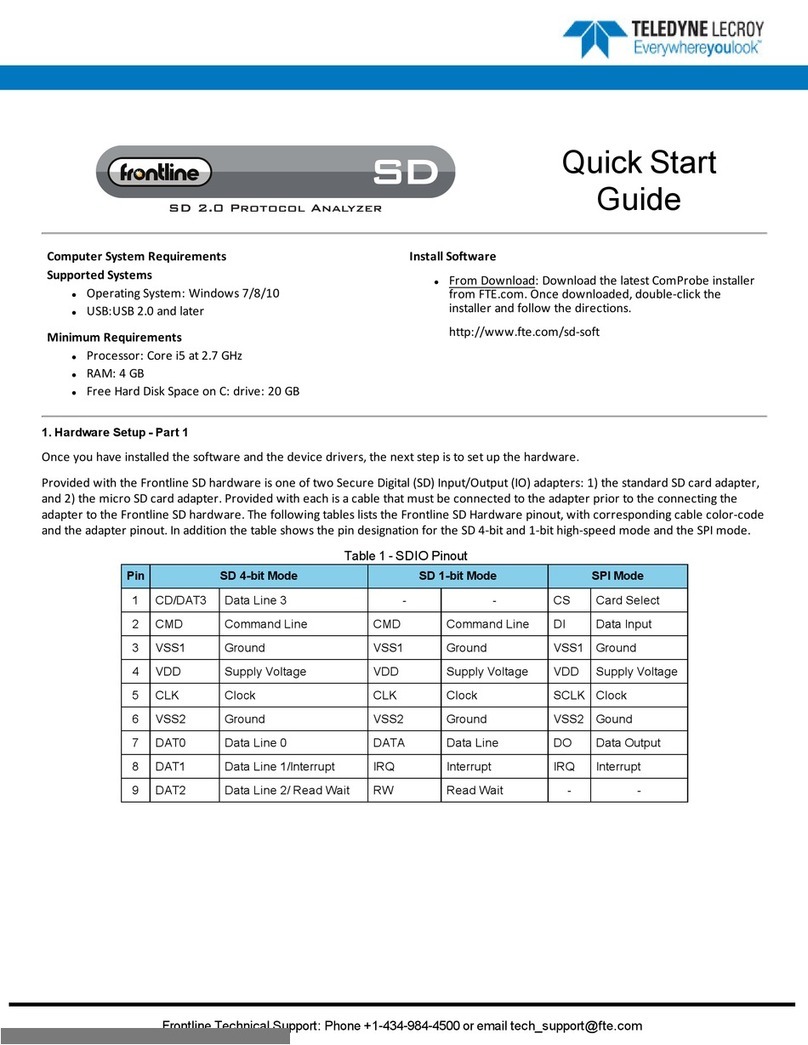
11
ENG
• Holding the Tonometer vertically
down, smoothly lower it down
by 2-3 mm. At this point, dynamic
force is actuated, which is
perceived as light vibration.
During the measuring process,
make sure that the buffer ring does
not touch the eyelid, but remains
2-3 mm above the eyelid surface.
Avoid slipping of the eyelid onto the cornea while
taking the measurement.
Attention! When the Tonometer is lowered too far down,
it produces a continuous single-tone beep, which stops
automatically when the device is raised high enough for
measuring.
!
• 1 or 2 seconds after lowering the Tonometer down, it
produces a beep indicating that the measurement is
completed, and the measured IOP value is displayed in
its window. The measuring process will continue until
the device is lifted away.
To end the process, lift the device up. At the moment
when the measurement is taken, the device produces
another beep, and the measured IOP value is displayed
in the window.
In case if the sound signal didn’t come offat all or came
offwith a delay of more than 3 seconds, the measuring
needs to be repeated.
Deactivate the Tonometer by shortly pressing the On/
Offbutton. Put the protective cap back on, with the
Tonometer rod up, and put the device back into its case.
•
•
Attention! If the positioning of the
Tonometer, the patient’s eyelid or eye, is
unstable during the measuring process,
the resulting reading may appear on the
display in a square frame. If this happens,
the measurement needs to be re-taken.
!
3.4. Requirements for Accurate Measuring Results
To obtain the most accurate IOP measurement results, the following
conditions must be observed:
• The Tonometer body should be posioned strictly upright.
Try to preserve the angular deviaon from vercal axis at 15 degrees
max., especially at the inial stage of the device applicaon.
• The Tonometer rod should be posioned at right angle against
the eye surface. To achieve that, align the Tonometer rod axis with
the geometric center of the eyeball.
• Smoothness and preciseness of movements during the measur-
ing process. These can easily be achieved when the hand holding
the Tonometer is resng against the paent’s head (forehead).