EME SHOCK MED SW2050 User manual

Rev.3 – 30/11/2020 SHOCK MED – SHOCK MED SP
INDEX PATIENT DATA ................................................................ .................................................. .. 21
CREATE A CARD ................................................................. .................................................21
OPEN a CARD ................................................................. .................................................. 22
EDITING a CARD ................................................................. ..........................................22
INFORMATION ON THE USER MANUAL ................................................ ........................1
WRITING CONVENTIONS ................................................................ ...................................... 1
WARRANTY................................................. .................................................. ..........2
NOTE................................................. .................................................. ..................2
WARNINGS ................................................. .................................................. ......3
SETTINGS ................................................................ .................................................. 22
SETTINGS................................................................ .................................................. .......23
HISTORICAL................................................................ .................................................. .................23
DEVICE MAINTENANCE ................................................................ ................................23
INTRODUCTION TO THE TECHNOLOGY ................................................ .......................5
Shock waves ................................................................. .................................................. ............. 5
Propagation velocity and diffusion of shock waves ................................................ ... 6
Shock wave generation systems ................................................................ ........................ 6
The Mechanism of Action........................................................ ................................................. 6
EXECUTION OF THE TREATMENT ................................................................. ................................ 24
MAINTENANCE................................................. ................................................ 24
OPERATION PROBLEMS ................................................................ .......................... 26
ELECTROMAGNETIC INTERFERENCE ................................................................ ................. 26
DIAGNOSTIC TECHNICAL DATA SHEET ................................................................ .......................... 27
TECHNICAL FEATURES ................................................ ................................ 28
IN GENERAL ................................................ .................................................. .......7
INTENDED USE.............................................. ................................................7
INDICATIONS ................................................ .................................................. ............ 8
COUNTER-INDICATIONS................................................................ ................................................. 9 APPENDICES........................................................ .................................................. ....... 29
Appendix A - ENVIRONMENTAL PROTECTION .......................................................... ................29
Appendix B – LABELS................................................................ ..........................................29
Appendix C – LIST OF THERAPEUTIC SUGGESTIONS ................................................ .30
Appendix D – ELECTROMAGNETIC COMPATIBILITY TABLES ..........................................31
PRELIMINARY NOTES................................................................ ................................................10
UNPACKING ................................................................ .................................................. .. 10
INSTALLATION ................................................................ .................................................. ..... 10
SEPARABLE COMPONENTS ................................................ ...................................... 10
CONNECTIONS ................................................. .................................................. ..... 11 SHOCKWAVE ACTUATOR REPLACEMENT PROCEDURE ................................ 33
TRANSMITTER REPLACEMENT PROCEDURE................................................................................ 34
DESCRIPTION OF THE APPLIANCE ................................................ ..........................12
FRONT PANEL................................................................ ................................................ 13
BACK PANEL ................................................ ........................................... 13
SEPARABLE COMPONENTS ................................................ ...................................... 13
USING THE MACHINE ................................................................ ..................................14
OPERATION................................................................. .................................................. 14
OPTIMAL USE ................................................................ ................................................. 14
FREE PROCEDURE ................................................ ............................................15
EDIT................................................................ .................................................. .............. 15
SAVE ................................................ .................................................. .................... 17
START ................................................ .................................................. .................... 17
LOAD................................................. .................................................. ................... 19
PATHOLOGIES................................................................ .................................................. ......20

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
USER MANUAL INFORMATION update production and manuals without the obligation to update previous
production and manuals unless these have implications for the safety of the
device.
This user manual is addressed to:
- user of the machine;
- owner;
- responsible;
- travel agents;
- installers;
- users;
- those in charge of maintenance.
The company considers itself relieved of any possible responsibility in the main cases:
- improper use of the machine;
- use contrary to specific national regulations;
- incorrect installation;
- power supply defects;
- serious shortcomings in the planned maintenance;
- unauthorized modifications and interventions;
- use of spare parts or materials not specific to the model;
- total or partial failure to comply with the instructions provided;
- exceptional events.
This document provides information for the installation and correct use of
SHOCK MED shock wave therapy devices.
It is an indispensable reference guide for the user: before installing and using
the machines it is essential to carefully read the contents of the manual and
always keep it at hand for quick consultation. If you require any further information, consult the EME srl company directly; it is
always updated on the best ways to use these machines and the optimal method
to provide the necessary assistance.
Failure to comply, even partially, with the recommendations contained therein may give rise,
in addition to malfunctions, to damage to the equipment, with invalidation of the warranty.
WRITING CONVENTIONS
On the other hand, only by scrupulously following the prescriptions and recommendations
provided by the manufacturer can you have the absolute certainty of obtaining maximum results
and benefiting, if necessary, from a fast and efficient technical assistance service.
Underlining is used to highlight certain sections of the document.
NOTE
The notes highlight some important information contained in the text.
The limits of this user manual are:
-the user manual can never replace adequate user experience; WARNING
Warning messages appear before operations which, if not observed, may cause
damage to the machine and/or its separable components.
-the instruction manual, for particularly demanding operations, can only
constitute a reminder of the main operations. ! ATTENTION !
The user manual is to be considered part of the equipment and must be
retained for future reference until the final dismantling of the equipment. The
instruction manual must be available for consultation near the machine and
stored correctly.
ATTENTION messages indicate operations or situations which, if not known or not
performed correctly, could cause problems for the user.
This user manual reflects the state of the art at the time of marketing and
cannot be considered inadequate just because it was subsequently updated
based on new experiences. The builder has the right to
FT05MI11 1

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
WARRANTY OPERATION: the possible inconveniences are mostly attributable to poor
maintenance or small technical problems which the user can effectively
intervene on.
EME srl guarantees the quality of its devices,when used in accordance with the
instructions provided in this manual , in the following ways: A simple phone call to the EME srl Technical Service can be of great help in
solving a problem.
-The warranty period of the camera body lasts for24 months from the date of
purchase. Instructions for packing and returning the appliance:
The components subject to wear, on which the standard 24-month camera body
warranty does not apply, are:
1. disconnect the power and connection cables with handpieces, applicator
devices, etc.;
-APPLICATOR handpiece
-Transmitter(s).
-INTERCHANGEABLE KIT
2. carefully clean and disinfect all separable components and parts of the
machine that have been in contact with the patient. For obvious hygienic
reasons, in order to guarantee adequate protection of the health of technical
staff (workplace safety directive, TUS 81/2008), equipment deemed
hygienically unsafe by the reception staff will not be checked;
Special note for the INTERCHANGEABLE KIT, whose warranty period lasts for6
months from the date of purchase, unless there is damage related to
inappropriate use or improper maintenance. 3. dismantle the separable components and any mechanical supports;
4. reuse the box and original packaging materials;
During the warranty period, at the company's discretion, defective products will be repaired
or replaced. 5. attach to the shipment the Assistance Request Form on which to note the
reasons for the revision request, the type of fault or malfunction: very useful
information that will facilitate the work of the technicians by significantly
shortening the repair times.
Under no circumstances will the appliance be replaced.
The warranty is not covered for malfunctions or damage resulting from :
- inadequate location, installation and implementation;
- incorrect use or use that does not comply with the provisions of this manual;
- improper or inadequate maintenance by the user;
- operation not compliant with the environmental specifications indicated for the product;
- unauthorized opening of the external packaging;
- tampering and/or unauthorized modifications;
- use of non-original separable components. The
guarantee is provided ex EME srl registered office.
NOTE
PRELIMINARY NOTES
−Installing the device does not require particular attention and is therefore simple and
immediate.
USE
-The keys shown on the display are touch and thanks to these it is possible to navigate the software.
−SHOCK MED devices are equipped with the automatic recognition function of the handpiece
connected to the output connectors.
−When MODIFYING THE PATIENT CARD, by clicking the ENTER button, the new data will be
saved on the selected card, deleting and overwriting the old ones which will no longer be
recoverable.
−The data modified during the treatment cannot be saved directly in the patient file,
it will be necessary to create a personalized treatment, as indicated in USER
PROGRAMS in order to create a personalized program.
−When CREATING THE CARD it is mandatory to insert the NAME field or the
SURNAME field and the treatment PROTOCOL of the pathology. Failure to enter
does not allow the patient card to be saved.
If a return shipment is necessary, follow the packaging instructions below and
attach a copy of the purchase receipt.
It is advisable to insure the shipment.
Before sending the machine due to a suspected malfunction, it is recommended to
carefully consult the MAINTENANCE and TROUBLESHOOTING chapters.
FT05MI11 2

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
−Every time the START button or the STOP button is selected the machine will emit a long
confirmation beep.
−When starting the treatment, after selection the START button is replaced by the STOP button
and vice versa.
−RESTORING THE FACTORY SETTINGS means deleting all patient records and
personalized protocols saved in the user memory; they will no longer be recoverable.
−Cards and programs deleted using the DELETE procedure will no longer be
recoverable.
−Once the SCREEN CALIBRATION has started it must necessarily be carried out, there is
no possibility of pressing ESC as otherwise indicated in the on-screen instructions.
MAINTENANCE
−For optimal use of the device and to guarantee its maximum performance, it is recommended
to correctly carry out maintenance within the recommended times and methods.
−Maintenance of the applicator kit, using the supplied brush, allows you to:
or
or
guarantee the patient the execution of an effective treatment over time and carried out in
conditions of absolute safety.
-Often check the integrity of the electrical power cable and the connection cable to
the handpiece/applicator: these must not be damaged or worn.
-It is a class B car in terms of emissions. The machine can be used in hospital or
outpatient settings, provided that due consideration is given to the fact that the
machine machine could cause disturbance to electronic devices located in the
immediate vicinity.
-Do not use the machine near HF SURGERY EQUIPMENT and rooms with RF
shielding of an EM SYSTEM for magnetic resonance, where the intensity of EM
DISTURBANCE is high.
-No modification of this appliance is permitted.
-The use of separable components, transducers and cables, other than those
specified or supplied by EME srl, could lead to greater electromagnetic emissions
or a decrease in the level of electromagnetic immunity of the device, with
consequent incorrect functioning.
Clean the barrel from bullet debris;
Lubricate the bullet slide barrel to avoid friction and air leaks USE
-Upon request it is possible to provide the machine's user manual on computer support.
-For safety reasons it must be loaded into the machineonly and onlythe software of
the relevant machine. In the event of software exchanges, the machine could
immediately block all its functions, requiring the intervention of the EME srl
technical assistance centre.
-Use different names for each custom protocol. To avoid entering the same name
for two different therapies, check the list of therapies before creating a new
personalized one.
-Before saving the customized protocol, check that the associated name has been
entered to avoid saving a therapy without a reference name.
-The operator is advised to familiarize himself with the typical rhythmic noises, linked to the
frequency and intensity of pressure delivered, which accompany the emission of shock waves.
-The equipment or system must not be used in proximity to other equipment and, if it is
necessary to use it near other equipment, the medical electrical equipment must be
observed to check normal operation in the configuration in which it is used.
-If the electromedical device, by interacting with another device, causes or receives detectable
interference, the user is invited to limit the interference by adopting one or more of the
following measures:
or
or
or
WARNINGS
PRELIMINARY NOTES
-The responsibility for damage resulting from inadequate packaging lies with the customer.Keep the
original packaging of the machine: it must be reused if returned to the company .
-Do not use the appliance in places where it could get wet.
-Carefully check the correctness of the connections according to the instructions provided
before operating the machine
-To avoid the risk of electric shock, this device must only be connected to power
networks with protective earth.
-Do not use separable components other than the original ones supplied: these
could damage the machine and void the warranty. If problems or installation
difficulties should occur, contact the EME srl technical assistance service.
-If you use an extension cord shared between the machine and other appliances, check that
the total current absorption of the connected appliances does not exceed the maximum
current allowed for that type of cable and that it does not exceed 15 A.
-Therapeutic suggestions are saved in the machine's fixed memory. These protocols
can be modified if necessary but it is not possible to save any changes made.
-The therapeutic suggestion protocols preloaded into the machine cannot be
deleted.
-It is not possible to define a suggested number of sessions to evaluate the effectiveness of the
treatment, since they are linked to the power delivered to the patient undergoing treatment. It
is the doctor's task to decide the number of therapeutic sessions to which the patient is
subjected based on the specific needs of the case, in order to be able to
Reorient or relocate the receiving device;
Increase the distance separating the appliances;
Connect the equipment into an outlet on a circuit different from the device(s)
causing the interference;
Contact the manufacturer or local technician for assistance.or
-Portable and mobile radio communications equipment can affect the operation of
the device.
-Transportable RF communications equipment (including peripherals such as
antenna cables and external antennas) should be operated no closer than 30 cm
(12 inches) to any part of the device, including cables
FT05MI11 3

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
specified. Failure to do so may result in degradation of the performance of this
equipment.
MAINTENANCE
-Handle the applicator handpiece with care: rough handling can negatively
influence its performance and characteristics.
-Unauthorized technical personnel are not allowed to open and/or disassemble the
handpiece/applicator for any reason: this tampering, in addition to damaging the
characteristics of the handpiece, immediately voids the right to the warranty.
-For no reason must the appliance be dismantled for cleaning or inspection purposes:
there is no need to clean the inside of the machine, and in any case this operation must
be carried out exclusively by specialized technical personnel authorized by EME srl.
-Do not use thinners, detergents, acidic solutions, aggressive solutions or flammable liquids to
clean the exterior of the machine and separable components. The use of these substances,
together with improper use of the separable components, in addition to irreparably damaging
the device and the electrodes, invalidates the warranty right.
-For optimal use of the device and to guarantee its maximum performance, it is recommended
that maintenance actions be carried out correctly within the recommended times and
methods.
-To correctly replace the fuses on the machine, follow the instructions below:
or
-When the BURST disturbance occurs, the device goes into protection, interrupting the therapy
and displaying the communication error on the screen. Once the disturbance is over, the
device returns to working properly.
-When the SURGE disturbance arrives at ± 2 kV, the display loses functionality, causing
the communication error message to appear on the screen, however the device
continues to function, whether it is in the standby condition or in therapy delivery. At this
point you only have the possibility to interrupt the therapy, only with the handpiece
button. Once the key therapy has been interrupted and the disturbance is over, it is
necessary to turn the device off and on again to be able to return to normal.
-When an electromagnetic compatibility disorder occurs, the device may react by
interrupting the delivery of the therapy, the display working correctly (also
displaying the communication error message) and giving the opportunity to pause
and restart the therapy. If this were to happen, it is necessary to pause or stop the
delivery of the therapy and then turn the device off and on again.
-When an electromagnetic compatibility disorder occurs, the device's display could
react by freezing, making it mandatory to turn the device off and on again. If this
should happen, you must turn the device off and on again.
PRELIMINARY NOTES
-The correct transport position of the machine requires that the appliance is moved
exclusively by gripping the curved profiles of the cover with both hands.
-The perfect functionality of the appliance is guaranteed in compliance with the installation and
use regulations indicated, only with separable components and original spare parts.
-If problems or installation difficulties should occur, contact the EME srl technical
assistance service.
-Before connecting the cable to the mains plug, check that the appliance has not
suffered damage during transport and make sure that the characteristics of the
electricity supply on the available socket satisfy the data on the plate on the back of
the machine.
-The electrical current supplied to the machine is VERY DANGEROUS. Before
connecting or disconnecting the power cable from the connector on the machine,
make sure you have previously disconnected it from the socket.
-For safety reasons, the power cable is supplied with a plug with a protective earth
connection.
-Only use a suitable grounded power socket.
-The connection of the appliance must only be done on compliant systems.
-If extension cords are used, check the presence and integrity of the protective earth
conductor.
-Connect the device directly to the wall socket, possibly without using extension
cords. Failure to comply with this warning could cause dangerous electric shocks to
people and alter the operation of the machine.
-The manufacturer is responsible for the fundamental safety, reliability and
performance of the device only if:
or The electrical system of the premises complies with the appropriate regulations;
unplug the plug and use a screwdriver to open the fuse holder, taking
care to insert the screwdriver into the cutout made in the fuse holder and
levering it outwards
insert a screwdriver into the two side holes of the tray for releasing the
fuses
remove the old fuses
insert a new fuse at a time by applying light pressure, to the left, with a
finger
push the tray back to fit it back into the slot.
or
or
or
or
−It is advisable to carry out periodic maintenance every two years, checking:
or
or
or
in order to guarantee the electrical safety of the device, make sure that it operates in the
guaranteed safety conditions. For this type of interest we recommend contacting a
qualified technical service or alternatively EME srl or one of its authorized centres.
−For correct maintenance of the applicator kit, carry outevery two weeksthe
cleaning procedure with a brush.Do not entercompletelythe brush inside the
barrel and do not force its insertion
OPERATIONAL PROBLEMS
-Only technical personnel authorized by the manufacturing company can access the
internal parts of the appliance.
-For repairs and further information it is necessary to contact EME srl or its
authorized service centers.
the intensity of any leakage currents;
the continuity, and therefore the integrity, of the earth
conductor; the correctness of the insulation resistance value
FT05MI11 4

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
or The device is used in accordance with the instructions for use. -For safety reasons, before carrying out any maintenance and cleaning operations
on the appliance, it is NECESSARY to turn off the appliance using the rear main
switch and disconnect the power cable from the socket.
-It is recommended to carefully clean the machine and the separable components
supplied before using it in contact with the patient.
-Cleaning and related disinfection must be carried out systematically before carrying out
the therapeutic treatment to which the patient is subjected.
-It is useful to draw the operator's attention to the need for periodic maintenance of
the handpieces/applicators, in particular:
➢checking the treatment head to detect any cracks that could allow the
entry of conductive liquid;
➢check the integrity of the cable and the handpiece/applicator connector.
-Do not spray or pour liquids on the external container of the appliance, on the
ventilation slots, in correspondence with the LCD TOUCH SCREEN display or on the fan
grate. Otherwise, overhaul the machine, EME srl will not be held responsible for any
damage caused following use of the machine in non-compliance with the conditions
listed above.
-Often check the integrity of the electrical power cable and the connection cables of
the applicators applied to the patient: these must not be damaged or worn.
-It is advisable to have the replacement of fuses carried out by personnel with adequate
technical training, in order to carry out the operation in safe conditions.
-Do not open the device: there arehigh electrical voltages which can be dangerous.
-Only technical personnel authorized by the manufacturing company can access the
internal parts of the appliance. For repairs and further information it is necessary
to contact EME srl or its authorized service centers.OPERATIONAL PROBLEMS.
−DO NOT OPEN the unit, there are HIGH ELECTRICAL VOLTAGES inside which can be
DANGEROUS .
USE
-Shock wave therapy treatments must be delivered, under the strict control of the
operator, to "conscious" patients, capable of interacting with the operator in the face of
the stresses transmitted by the machine.
-If the patient's pain threshold does not allow the delivery of the maximum energy density
expected, use the maximum tolerated level. Achieve the maximum energy expected, or
tolerated, in the protocol by increasing the energy density every 100 pulses.
-Before turning on the generator, adjust the ring nut to the correct value of the mains
voltage in use in the room where the treatment will be provided so as not to cause
malfunctions of the machine.
-NEVER OPERATE THE HANDPIECE BEFORE HAVING CORRECTLY INSERTED THE
DISPENSING HEAD. DAMAGES SUFFERED BY THE DISPENSING GUN UNDER THESE
CONDITIONS ARE NOT COVERED BY THE WARRANTY.
-To obtain perfect recognition of the handpiece connected to the output channel, it is strongly
recommended to connect/disconnect them when the delivery of treatments is interrupted.
-Once delivery of a program begins, the toolbar buttons are disabled; the only
operation allowed is stopping dispensing by pressing the STOP button.
-In order to guarantee the operation of the machine in conditions of absolute safety for
the patient, it is recommended to subject the machine to a cycle of periodic checks (at
least every 2 years) to be carried out by an authorized EME technician.
-The use of the device in the presence of flammable anesthetic mixtures and oxygen-rich
environments is absolutely prohibited. In case of failure to comply with the instructions
provided, EME srl will not be held responsible for any accidents.
-It is absolutely forbidden to cover the compressor's ventilation slots: such an action
may not allow the machine to work in safe conditions. In case of failure to comply
with the instructions provided, EME srl will not be held responsible for any
accidents.
-It is important to draw the operator's attention to the need to verify the
correctness of the electrical installation of the appliance before operating the
mains switch.
-It is advisable to suspend the therapeutic treatment if any disturbances appear
during its provision.
-Before each treatment, carefully clean and disinfect all separable components and
parts of the machine that have been in contact with the patient, in particular the
shock wave transmitters.
MAINTENANCE
-It is absolutely forbidden to remove the electrical/pneumatic connector of the applicator
without first having discharged the pneumatic circuit. Then TURN OFF the device with
the main switch and wait 10 seconds for the pneumatic discharge. This procedure is
introduced to safeguard the integrity of the O-Ring inserted into the connector.
INTRODUCTION TO TECHNOLOGY
The shock waves
From a physical point of view, shock waves are defined as high-energy acoustic
waves. In particular,they are pressure pulses that generate a direct mechanical
force, with the main objective of transferring energy to the body tissues for
stimulate its reparative processes.
The shock wave should not be confused with the ultrasound wave which is
frequently used for both diagnostic and therapeutic purposes. Unlike the
ultrasonic wave, the shock wave has an impulse pattern and generates much
higher pressure values, on average 1000 times higher.
FT05MI11 5

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
The shock waves used in therapy are particular acoustic waves with characteristics
specified at an international level (DIGEST). To allow users to make reliable and useful
measurements for therapy and research, the most representative parameters of the
acoustic field were chosen, in agreement with the International Society for Medical
Shockwave Therapy (ISMST) and the manufacturers of shock wave equipment:
characteristics of the medium and are inevitably affected by the differences in
density and impedance of the skin, fat, muscles and bone.
Shock wave generation systems
There are different types of shock wave therapy equipment which are
distinguished by the technological methods with which these waves are
generated. In general a shock wave generator is made up of:
-therepressure (measured in MPa, 1MPa=10 bar i.e. approximately 10 atmospheres):
SHOCK MED is able to generate up to 5 bar of pressure and SHOCK MED SP up to 4 bar
of pressure;
- adevice to cause pressure stroke;
- onewater chamber to concentrate the shock wave energy into the focal volume
-thereenergy flux density (measured in mJ/mm2);
-L'power (measured in mJ);
desired or aDomed rubber membrane to close the shock wave output window.
-thedimensions of the focal volume, defined by convention at 50% of the maximum
pressure.
This membrane acts as a means of coupling with the skin of the patient to be treated or
as a ballistic system consisting of a spring-loaded metal applicator (radial shock waves)
.
Propagation speed and diffusion of shock waves
In the medical field, shock waves are therefore produced through a strong
and immediate increase in pressure inside a water chamber or a ballistic
system obtained.
The speed of propagation of a shock wave, as for any acoustic wave, depends above all
on the medium in which it is transmitted and on the intensity of the shock wave itself.
Specifically, in a ballistic or radial system the shock wave is generated in a
pistol-shaped handpiece in which the end is closed by a metal "cap" against
which it is launched, using compressed air at 5 bar pressure a steel bullet. The
collision generates a shock wave which spreads through the metal cap,
expanding radially onto the skin and into the first underlying layer of tissue.
Biological structures such as cell walls, whose thickness is comparable to a few
molecular layers, are therefore subjected to very high pressure gradients when
shock waves transit.
The mechanical properties of biological media subjected to shock waves, such as
elasticity and compressibility, influence the transmission of acoustic waves,
determining their propagation speed. The Mechanism of Action
When shock waves pass through a fluid they generate multiple pressure differences
which give rise to the formation of gas bubbles. A subsequent shock wave that hits the
bubbles thus formed gives rise to a violent implosion which forms a jet of liquid which
will hit the tissue to be treated. In the face of such lesions, a series of desired biological
events are generated which trigger different types of responses depending on the
tissue affected.
The mechanism of action in musculoskeletal tissues is very complex and still
under in-depth study. Shock waves act differently depending on the
pathological tissue they treat (bones, soft tissues, skin). In general they
stimulate the activation of natural biological repair processes.
However, the mechanism of action of shock waves seems to be attributable to two
main effects:
In particular, an osteogenic and a vascular type reaction were observed in the
bone tissue, while in the soft tissues an anti-inflammatory and analgesic effect
occurred, as well as a vascular response.
1. direct physical-mechanical effects:
the so-called "cavitation effect" and micro-streaming resulting in the formation
of new blood vessels to increase the local blood flow and the production of
new cells to speed up the repair of micro-lesions and improve tissue trophism;
The diffusion of the acoustic wave in the tissues follows the physical laws of the
acoustic waves of transmission, reflection and absorption, which are linked to the
FT05MI11 6

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
2.indirect biological effects induce: INTENDED USE
the reduction of pain transmission by stimulating nerve endings and releasing
substances that modulate its perception; the vascularization that produces
biomolecular modifications. SHOCK MED and SHOCK MED SP is an electro-medical device that provides
therapeutic treatments in which the shock wave is used with the aid of a special
handpiece/applicator.
IN GENERAL
Shock waves are acoustic waves that transfer high energy, transmitted through
the surface of the skin and spread radially throughout the body, in the area of
pain. The body responds with an increase in metabolic activity in the area of
application, favoring the reduction of inflammation caused by a pain-relieving
action induced by the local release of endorphins, thus stimulating and
accelerating the healing process.
EME srl has recently developed a complete series of devices, accessories and
equipment, designed and built according to the highest quality standards,
adopting cutting-edge technologies in full compliance with current directives and
standards.
Particular attention was paid to design, ease of operation, functionality and
safety. The result is a compact unit, equipped with a modern design, capable
of proposing an extremely logical operating sequence, supported by a clearly
legible display.
The use of SHOCK MED is reserved for operators such as physiatrists, physiotherapists and
pain therapists, who, by virtue of their professional training, offer the guarantee of
adequate use and total safety for the patient.
The multiple possibilities of therapeutic applications, together with the guarantee of
safety for the patient and the therapist himself (the unit complies with international
regulations), make the machine a high quality piece of equipment.
The operator, in fact, must be appropriately qualified and have carefully studied
the contents of the user manual in order to use the device; or, it must operate
under the supervision of a healthcare professional adequately qualified to use the
machine, able to understand the advantages and limitations of the therapy and to
work in safe conditions for the person undergoing treatment.
These machines have been designed and manufactured so that their use, if it occurs under
the conditions and for the intended uses, does not compromise the health and safety of
patients, users and third parties, taking into account the benefit brought to the patient. This machine can be used in a hospital or outpatient setting, provided it is
used by qualified personnel in this regard and in accordance with what is
stated in the user manual.
These machines are not reserved for diagnosis, prevention, monitoring, compensation
of injury or handicap, replacement or modification of the anatomy, control of
conception, support/support of vital functions but they allow the treatment of
particular pathologies and the reduction of the disease.
SHOCK MED are radial shock waves, as the shock wave is generated using a
special pistol-shaped handpiece, the barrel of which is closed at the end by a
metal element against which a steel bullet is launched by compressed air (up
to 5bar pressure).
No special intervention is required in the event of a medical device failure, but
only normal maintenance/repair work. A shock wave is thus generated which spreads, expanding radially in the skin and in
the first underlying layer of tissue, or in a focused manner (depending on the
transmitter used). The measurement of the penetration depth varies from 4 to 7 cm.
FT05MI11 7

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
INDICATIONS The term tendinopathy refers generically to a painful condition that develops in or
around the tendon when subjected to overuse. When this involves the shoulder we
speak of "insertional tendinopathy of the rotator cuff", i.e. an inflammation of the
tendons of some of the muscles responsible for shoulder movement, such as the
supraspinatus, infraspinatus and teres minor.
The main applications are used in the following fields: Orthopaedics, Rehabilitation and
Sports Medicine.
The shock wave method is the treatment of choice in chronic insertional
tendinopathies, characterized by poor vascularization of the osteotendinous
junction, where physiotherapeutic treatment (infiltration and laser therapy)
has proven ineffective.
The most common tendinopathies are those affecting the supraspinatus and
infraspinatus, while those involving the subscapularis are less common.
Impingement syndrome is a pathology that can lead to the gradual
degeneration of the supraspinatus muscle tendon. In impingement syndrome,
during the lifting movement of the arm and in the phase of returning to the
initial position, there is a compression of the supraspinatus muscle tendon,
which generates pain. Narrowing of the subacromial space due to anatomical
causes or biomechanical alterations of the shoulder (e.g. imbalance between
the rotator cuff muscles, misuse of the shoulder, chronic tension, repeated
microtraumas, etc.).
The list of the main treatable pathologies includes: Elbow:
epicondylitis and epitrochleitis
Epicondylitis and epitrochleitis are two inflammatory pathologies due to a
degeneration of the tendon insertion of the epicondylar muscles, i.e. the extensor
muscles, and the epitrochlear muscles, i.e. the flexor muscles, of the elbow.
These pathologies arise as a consequence of tendon overload due to continuous
stress on the insertion of the aforementioned muscles. Impingement syndrome can lead to gradual degeneration of the tendons and,
over time, even to their rupture.
Lateral epicondylitis, also known as "tennis elbow", is a syndrome that occurs
in subjects who repeatedly perform pronation and supination movements of
the forearm in a condition of complete extension of the elbow. It manifests
itself as lateral elbow pain during wrist extension and high intensity pain
during movements performed to grasp objects.
Knee: tendinopathies of the patellar or quill
The patellar tendon connects the lower part of the patella with the upper part of the
tibia and its function is to transmit the contraction of the quadriceps muscle to the tibia
to extend the leg.
Even in the case of epitrocleitis, or "golfer's elbow", there is tendon
degeneration due to incorrect use of the articulation of its tendons. Patellar tendinopathy is a knee disorder that affects the part of the tendon
underneath the kneecap. In the majority of cases, the resulting pain is caused
by chronic and continuous stress on the patellar tendon which leads to small
lesions, which can degenerate over time.
The effectiveness of shock wave treatment for the indicated pathologies appears
to be due to the neovascularization of the tendon-bone junctions; in fact, by
improving the blood flow in the tissues, there is an increase in cell proliferation
which leads to the regeneration of tendon and bone tissues. Patellar tendinopathy is a very frequent pathology and the subjects most at risk
are those who practice sports activities in which the ischio-perineotibial muscles
are subjected to continuous stress.
Shoulder: insertional tendinopathies, impingement
TENDONS are robust fibrous structures, with a mother-of-pearl color, that connect
muscles to bones. These important anatomical structures therefore function as
real connections, capable of transforming the force generated by muscle
contraction into movement.
Pubis: adductor tendinopathies (pubalgia)
The adductor muscles are large muscles that allow a limb to be brought closer to the
median axis of the body.
Like all anatomical structures, tendons can also undergo degenerative
phenomena over time. Furthermore, tendons have long healing times and a
marked propensity to evolve into a state of chronic inflammation.
Adductor tendinopathy, also known as "hip adductor syndrome" or simply
"pubalgia", particularly affects the pubic insertion of the adductor longus and
the pectineus muscle. It can be caused by microtraumas
FT05MI11 8

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
repeated or following an episode of muscular distraction not correctly treated. -Acute soft tissue/bone infection
-Epiphysiolysis at the focal point
-Patients with active implantable devices
Insertional adductor tendinopathy is typical in subjects who practice sports that
require a high frequency of explosive actions and is generally caused by careless
or incomplete preparation of the athlete. -Brain, spinal cord, large nerves at the focal point (neurocranium, spine,
ribs)
In initial cases, the pain appears upon awakening and at the beginning of sporting activity
and then disappears once the athlete has warmed up. In the most severe forms, the pain
does not ease following muscle warming but tends to worsen to the point of compromising
the continuation of the activity.
-Severe osteoporosis. It should be noted that in cases of severe osteoporosis or
advanced bone necrosis, shock wave therapy cannot be performed.
-Surrounding metal prostheses
-Use of vasoconstrictor drugs
Relative
-Rotator cuff tear
Ankle: Achilles tendinopathies, calcaneal apophysitis.
The Achilles tendon is the largest tendon in the human body, capable of withstanding a load
capacity of up to approximately 12.5 times body weight and which connects the calf muscles
to the heel.
Achilles tendinopathy involves inflammation of the Achilles tendon and is
generally caused by an injury that occurs during running or playing sports.
-Tendinopathies associated with severe glenohumeral arthritis or secondary to
capsular-ligament instability.
Apophysitis is a pathological inflammatory state of an apophysis, i.e. a bony
prominence. Calcaneal apophysitis, or Sever's disease, is an inflammation of
the calcaneal apophysis, where the Achilles tendon inserts. It mostly appears
following a sudden increase in workloads in children aged between 9 and 15.
The cause for this pathology appears to be the tension exerted by the Achilles
tendon on the calcaneal tuberosity which, not yet being completely ossified
during adolescence, is pulled away from the calcification nucleus, inflaming
the growth cartilage. A contributing factor may be the repetitive stress caused
by the impact of the heel on the ground during running and jumping.
-Pernicious primary diseases
-Epiphysiolysis at the focal point
-Blood clotting diseases and use of anti-coagulants
-Lung tissue at the focal point
Side effects
-Hematomas and/or petechiae particularly with high energy pulses
(>0.60mj/mm2);
-Edemi
COUNTER-INDICATIONS -Flare-up of symptoms in the following 2-3 days which disappear on their own or
with cryotherapy and painkillers.
Absolute
-Pregnancy
-Coagulation disorders
-Presence of neoplasms or growth nuclei in the application field
-Demyelinating polyneuropathies
-Infectious tenosynovitis
-The proximity of the lung parenchyma to the scope
FT05MI11 9

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
PRELIMINARY NOTES INSTALLATION
The installation of shock wave therapy devices does not require particular
attention and is therefore simple and immediate.
UNPACKING
SHOCK MED shock wave therapy devices are packaged and prepared for
shipping with their box, complete with filling, designed for safe storage and
transport.
Once the device has been positioned, block the wheels with the appropriate brake to
prevent involuntary movements.
The environmental characteristics recommended for the installation of SHOCK MED are the
following:
To unpack the machine, place the box on a flat, solid surface and remove the
top polystyrene part. Remove the appliance carefully. −ambient temperature: from +10° to +35°C;
−relative humidity: 10% to 80% non-condensing;
The machine and the separable components are wrapped in a protective
transparent polyethylene sheet and the package always contains:
- n.1 user manual;
- n.1 mains power cable;
- n.2 reserve fuses (see technical characteristics);
- n.1 SWT Shock-Med applicator handpiece;
- n.1 9 mm multi-focused transmitter;
- n.1 15 mm focused transmitter;
- n.1 15 mm multi-focused transmitter;
- n.1 lubricated brush;
- n.1 gel 1000 ml;
- n.2 interchangeable applicator kits (one is the one inserted in the gun);
- n.1 actuator removal wrench tube
- n.1 actuator removal wrench handle
- 1 knob wrench for fixing the ring nut.
−avoid direct exposure to sunlight, chemical products, high intensity
magnetic fields and vibrations;
−avoid using in close proximity (<0.30m) to wireless RF communication
devices
SEPARABLE COMPONENTS
The appliance is supplied with a mains power cable and is compatible with the
following kit of separable components:
Description Supplied Optional
Shuko plug power cable 1
Pair of FUSES (see table) 1
Lubricated brush 1
User manual 1
Shockwave gun 1
1000 ml gel bottle 1
15mm focused transmitter 1
9 mm multi-focused transmitter 1
15 mm multi-focused transmitter 1
Check the contents of the package. If any element is missing, immediately
contact the authorized EME srl dealer.
2one is that
inserted into the
pistol
Interchangeable KIT for applicator
Actuator removal wrench tube 1
Actuator removal key handle 1
Ring fixing knob wrench 1
Handpiece case + shaped polystyrene 1
Applicator including 15 mm transmitter X
FT05MI11 10

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
Description Supplied Optional
15mm focused transmitter X
9 mm multi-focused transmitter X
15 mm multi-focused transmitter X
35mm focused transmitter X
35mm focused transmitter wrench X
Interchangeable applicator kit X
The separable components that can be replaced by the RESPONSIBLE
ORGANIZATION and which can affect the conformity of the EM EQUIPMENT:
Pneumatic-electric hybrid cable: 1 pneumatic tube and 3 electric cables. The cable
length must be less than 3m.
The assembly of the separable components is simple and intuitive: should
problems or installation difficulties arise, contact the EME srl technical
assistance service.
We recommend using the gel marketed by Fiab, model G009, or an equivalent
gel.
CONNECTIONS
Connecting the shock wave handpiece/applicator is simple: you need to plug its
connector into the appropriate socket on the front panel of the machine.
In the rear part of the machine there is the integrated mains power module,
which includes the three-pole connector for the power cable, the removable
fuse holder with two fuses (see technical characteristics) and the two-pole
main switch.
Insert the three-pole female plug of the power cable into the integrated
module, checking that it is perfectly inserted inside the connector.
After having checked the correct installation and assembly, turn on the main
power switch and check that the display turns on correctly.
FT05MI11 11

SW2050 - SW2051 SHOCK MED - SHOCK MED SP
DESCRIPTION OF THE APPLIANCE
TOUCH color display
SCREEN Back panel
HANDFUL
APPLICATOR
Front panel
FT05MI11 12

SW2050 SHOCK MED
FRONT PANEL APPLICATOR
Transmitter
Tightening ring nut
Connector
link for the
APPLICATOR handpiece
BACK PANEL TUBE WITH HANDLE
including the actuator
Removal key
actuator
Tray
fuse holder Socket for trolley
power cable (not Connector for
connection of
USB pendrive
Wave actuator
shock
Switch
ON/OFF
general
in use)
Three-pole socket
for cable
diet
USB connector,
cart data connection
(not in use)
Knob wrench
for transmitters
Pedal connector
(not in use)
SEPARABLE COMPONENTS
TRANSMITTERS
Lubricated brush
Transmitter
multi focused
15 mm
Transmitter
focused 15 mm
Transmitter
multi focused
9mm
FT05MI11 13

SW2050 – SW2051 SHOCK MED – SHOCK MED SP
USE OF THE MACHINE The standard therapeutic suggestion protocols are saved in an additional fixed
internal memory of the machine. This memory is not user-manageable: data
cannot be deleted or formatted. To make any changes made available, you
need to store them on one of the alternative media by creating a customized
protocol.
This chapter will provide important information on the correct use of the
SHOCK MED shock wave therapy device.
All the control functions and the entire functional structure of the machine are
managed and coordinated by a microcontroller: in addition to the task of making the
application programs already stored available, it allows for optimal and safe use of the
device in a personalized way.
OPTIMAL USE
After having installed and positioned the machine according to the instructions
provided in the previous chapters, and having applied the cable for connecting the
handpiece to the appropriate connector, insert the power plug into the wall socket
(230Vac) and activate the device bringing the main ON/OFF switch on the rear
panel to the “ON” position.
The dialogue interface with the user is carried out by a large backlit graphic liquid
crystal display (LCD) TOUCH SCREEN: all the operational messages of interest to
the operator are displayed on it, as well as the functional status of the machine
during normal therapeutic activity, any error messages. This operation prepares SHOCK MED for operation, causing the backlit LCD
display to turn on, signaling that the device is ready to operate.
The following paragraphs illustrate the operations that must be carried out by the
operator to make the most of the potential and technical peculiarities of the
SHOCK MED device.
The different options are covered, from the selection of a pre-stored program
for setting a specific therapy, up to the determination of the correct working
parameters for a "personalized" application.
The shock wave acoustic radiation delivered by SHOCK MED has a health purpose,
therefore it cannot be minimized.
There is therefore no need for protection means in this sense for the patient, who receives
treatment for healthcare purposes, nor for the operator, who is not in any way affected by
the acoustic radiation emitted by the applicator handpiece.
OPERATION
fig. 1
The SHOCK MED shock wave therapy devices have a control console optimized
according to the specific sector of use and the type of operation for which they
are intended. The LCD display will light up, highlighting a presentation screen (fig.1).
followed by a PASSWORD ENTRY screen:
All operating parameters are managed and controlled in real time by a
sophisticated microcontroller electronic circuit, with clear representation and
signaling of the various functions via a backlit LCD touch-screen display (located
on the machine) and appropriate acoustic signals.
1. type the login PASSWORD
orin the event of an incorrect password, a warning information appears
the user to retype the password
SHOCK MED gives the possibility of saving personalized programs and patient
cards in the memory medium calledUSER MEMORY in which both personalized
protocols and patient records can be stored.
2. once you have entered the correct password you will access the main
screen where you can select the desired operating mode from the 4
available.
FT05MI11 14

SW2050 – SW2051 SHOCK MED – SHOCK MED SP
The password has been set by default to1 2 3 4 5: to type it, simply press the 5
numeric buttons in sequence and then the OK button. Entering the code
prepares SHOCK MED for operation.
FREE PROCEDURE
By pressing the FREE PROCEDURE button a screen appears (fig.3) where you
can:
This code can be modified by the user (see SETTINGS - DEVICE MAINTENANCE -
GENERAL section). -modify the processing data, proceeding as indicated in MODIFICATION;
-save any modified parameters by proceeding as indicated in SAVE;
-upload a personalized treatment as indicated in UPLOAD;
-start treatment, following the START procedure.
fig. 2
On the home screen you can (Fig.2):
-access the FREE PROCEDURE section
-access the PATHOLOGIES section
-access the PATIENT RECORDS section
-access the SETTINGS section by clicking on the button at the bottom
right.
fig3
EDIT
In this section it is possible to modify the values of the treatment parameters set
by default in the machine in order to create customized programs.
The operation of each button will be described below.
1. Click on the parameter to be modified, the modification screen appears where
the name of the parameter to be modified is shown and it is possible to
increase or decrease the value using the + or – buttons or by scrolling the
cursor to the right or left until reaching the value desired;
Before starting any treatment it is very important to connect the handpiece to
the appropriate connector on the front panel of the machine.
FT05MI11 15

SW2050 – SW2051 SHOCK MED – SHOCK MED SP
or Click onHE CONFIRMS(green tick) to save the set value of the
parameter and return to the main screen;
or Click onBACKWARDS(x gray) to cancel the parameter
modification operation, you return to the main screen without
having made any changes.
4. For the parameterMODEchange the mode using the + or – buttons until
you reach the desired one. This parameter represents the delivery
mode of the shots emitted by the applicator handpiece during the
treatment, the choice is between 5 emission modes: SINGLE, CONTINUOUS, BURST,
CONTINUOUS AUTO, BURST AUTO.
fig 4
2. For the parameterHITS(number) increase or decrease the value using the + or –
buttons or by scrolling the cursor to the right or left until the desired value is
reached (figure 4). It is possible to vary the URTI parameter between 10 and
10000.
or Click onHE CONFIRMS(green tick) to save the set value of the
parameter and return to the main screen;
or Click onBACKWARDS(x gray) to cancel the parameter
modification operation, you return to the main screen without
having made any changes.
fig 6
-By selecting the AUTO BURST and BURST delivery modes, other
editable parameters appear on the screen (figure 6): SHOTS, i.e. the
number of pulses that make up the burst (or train of pulses) and
PAUSE (ms) (only in the AUTO BURST mode), i.e. the pause between
consecutive bursts.
-By selecting the SINGLE delivery mode, the FREQUENCY parameter
cannot be modified.
or Click onHE CONFIRMS(green tick) to save the set value of the
parameter and return to the main screen;
fig 5 or Click onBACKWARDS(x gray) to cancel the parameter
modification operation, you return to the main screen without
having made any changes.
3. For the parameterFREQUENCY(Hz) increase or decrease the value using the + or
– buttons or by scrolling the cursor to the right or left until the desired value is
reached (figure 5). It is possible to vary the FREQUENCY parameter between 1
and 20 Hz.
FT05MI11 16
SW2050 – SW2051 SHOCK MED – SHOCK MED SP
or Otherwise, click onBACKWARDS(x gray) to cancel saving the
therapeutic program, the screen with the modified treatment
parameters will reappear;
4. To start the saved customized program, proceed as described in the
START section.
When saving a new customized program, the software performs a check on
the programs already present in the database.
If the therapeutic program has an already existing identifying name, the
impossibility of saving the data with that specific name will be indicated unless you
choose to overwrite the therapy:
fig 7 or
or
ClickYESto proceed with overwriting the therapy;
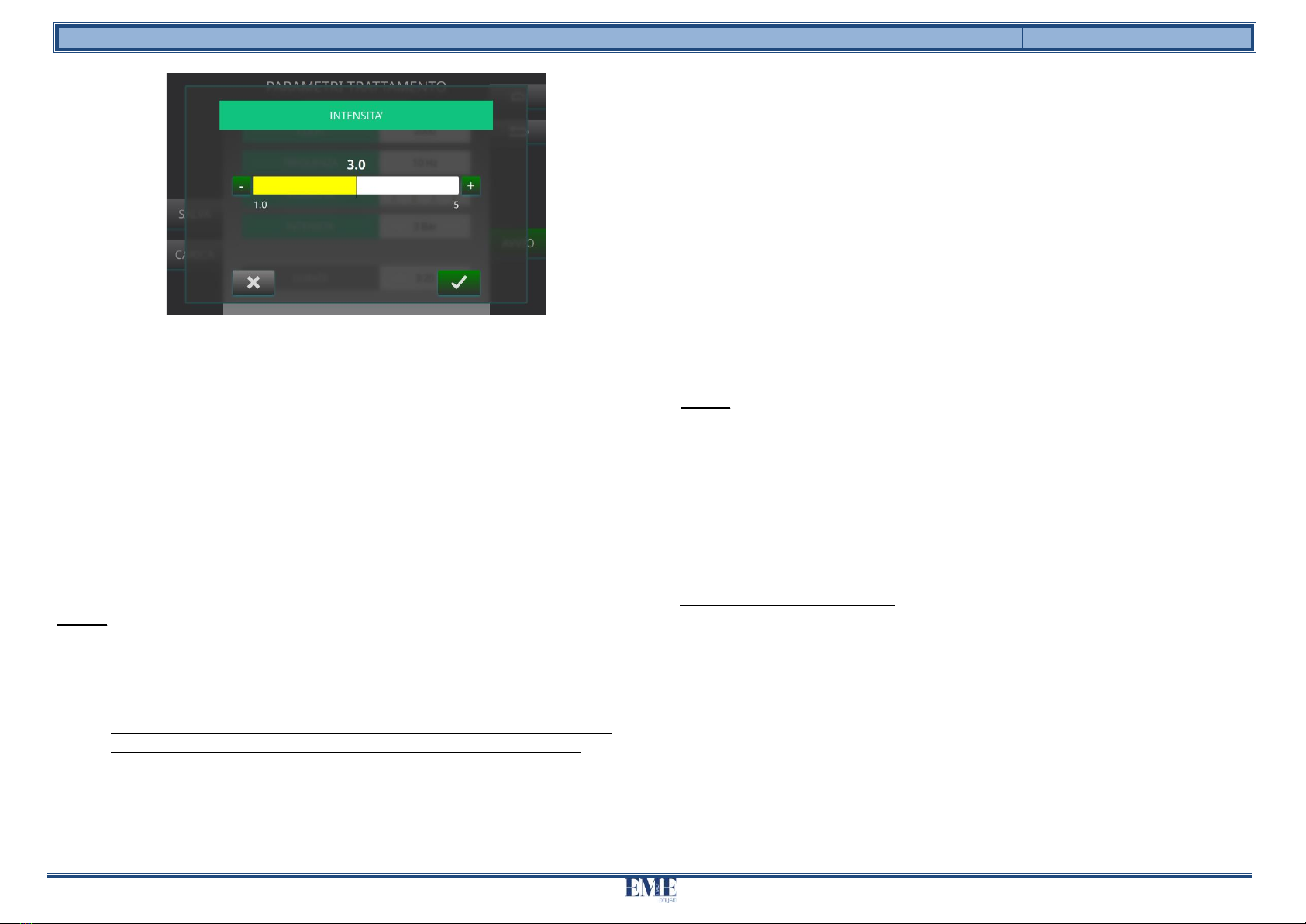
5. For the parameterINTENSITY'(Bar) increase or decrease the value using the + or
– buttons or by scrolling the cursor to the right or left until the desired value is
reached (figure 7). It is possible to vary the INTENSITY parameter between 1.0
and 5.0.
ClickNOto cancel overwriting the therapy and enter a new name to
assign to the created therapy program.
START
or Click onHE CONFIRMS(green tick) to save the set value of the
parameter and return to the main screen;
By clicking START it will be possible to start the treatment depending on the
selected delivery mode.
or Click onBACKWARDS(x gray) to cancel the parameter
modification operation, you return to the main screen without
having made any changes.
Connect the applicator handpiece into the appropriate connector on the front panel of the
machine.
-If you start the treatment without having connected the handpiece, an error message
“HANDPIECE ERROR” appears on the screen which prevents the start of the
treatment.
6. For the parameterDURATION(minutes) is a parameter that cannot be
modified directly but varies automatically as the number of IMPACTS, the
frequency and the set MODE vary. AUTO BURST delivery MODE To start
delivering a treatment:
SAVE
To save any changes made to the parameters and store a personalized
therapy program: 1. Place the applicator handpiece on the part to be treated;
2. proceed with the emission by initially pressing the trigger on the handpiece (or
the pedal): this enables the emission autonomously;
3. At the end of the burst, the next pulse train is emitted automatically
after a pause time set directly by the operator;
4. select STOP to end the treatment early,
1. Click the buttonSAVE;
NB: It is possible to save the protocols only in the INTERNAL MEMORY of the
machine. It is not possible to store personalized treatments on the USB.
2. Type the name to assign to the created therapeutic program on the
virtual keyboard; 5. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
3. Click onHE CONFIRMS(green tick) to continue with the program saving
operation;
FT05MI11 17
SW2050 – SW2051 SHOCK MED – SHOCK MED SP
CONTINUOUS AUTO delivery MODE To
start treatment:
1. place the handpiece on the part to be treated
2. touch the START button
CONTINUOUS delivery MODE To
start treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
3. proceed with the emission by initially pressing the trigger on the handpiece
(or the pedal): this enables the autonomous emission of a succession of
pulses set for the delivery of the treatment.
3. proceed with the emission by continuously holding down the trigger on the
handpiece (or the pedal): this enables the emission in rapid succession of the
pulses set for the delivery of the treatment;
4. to suspend delivery, press the applicator trigger (or the pedal); 4. to suspend delivery, remove pressure on the applicator trigger (or on
the pedal);
5. to resume treatment, press the applicator trigger (or the pedal) again;
5. to resume treatment, keep the trigger pressed continuously, as already
seen previously;
6. select STOP to end the treatment early,
6. select STOP to end the treatment early,
7. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button. 7. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
BURST delivery MODE To
start treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
SINGLE delivery MODE To start
treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
3. proceed with the emission by continuously pressing and holding the trigger (or
the pedal) on the handpiece: this enables the emission of the first train of
pulses (burst);
3. to proceed with the emission, press the trigger on the handpiece (or the pedal): this
enables the emission of a single shot (or the pedal);
4. At the end of the burst, the next pulse train is emitted automatically
after a pause time set directly by the operator;
4. to fire new shots, press the trigger repeatedly;
5. select STOP to end the treatment early,
6. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
5. To suspend delivery between one burst and another, remove pressure on the
applicator trigger (or on the pedal);
6. to resume treatment, keep the trigger pressed continuously, as already
seen previously;
7. select STOP to end the treatment early,
8. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
FT05MI11 18
This manual suits for next models
1
Table of contents
Other EME Medical Equipment manuals
Popular Medical Equipment manuals by other brands

GCE
GCE ELITE ELH BS PROBE Instructions for use

Acqua Brevetti
Acqua Brevetti BRAVADOS Installation and operating manual

medi
medi Lumbamed facet Instructions for use

Stryker
Stryker 1027 Maintenance manual

Kyoto Kagaku
Kyoto Kagaku BREAST FAN instruction manual
Seers Medical
Seers Medical PTX4200 operating instructions