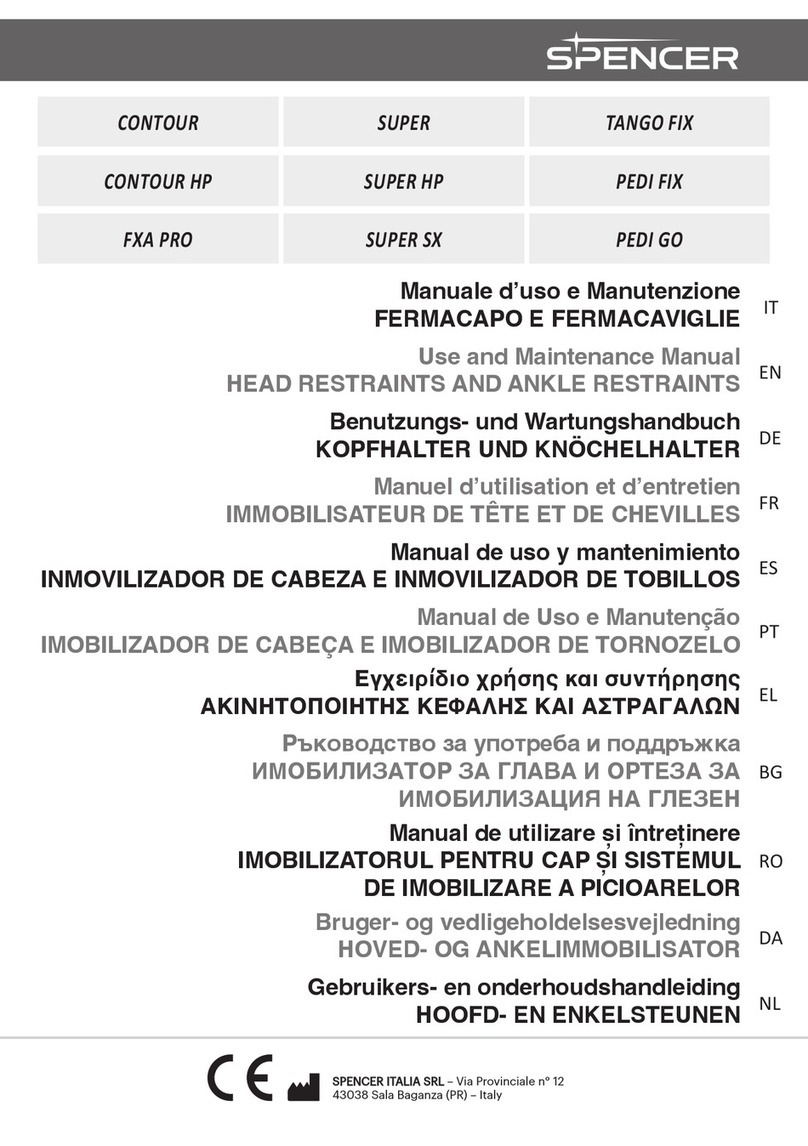
6
Stoccaggio
The device should not be exposed to or come into contact with any source of combustion or inflammable agents. Store in a cool, dry,
dark place and do not expose to direct sun.
Do not store the device underneath any heavy objects which could cause structural damage.
Store and transport the device in its original packaging. Failure to do so makes the warranty void.
Storage temperature: from -10°C to + 60°C
Maintenance/cleaning
Spencer Italia S.r.l. disclaims any liability for any damage, direct or indirect, which is a result of improper use of the product and
replacement parts and / or otherwise of any repairs made by an entity other than the authorized Spencer service centres; this will also
invalidate the warranty.
The operator must always wear adequate personal protection such as gloves and mask etc. during all checking, maintenance and
cleaning procedures.
Establish a maintenance program and periodic testing, identifying an employee responsible for overseeing. The person to whom the
ordinary maintenance of the device is entrusted must ensure the basic requirements foreseen by the manufacturer in the user’s manual.
The frequency of inspection is determined by factors such as legal requirements, the type of use, frequency of use, environmental
conditions during use and storage.
Spencer Italia S.r.l. disclaims any liability for any damage, direct or indirect, that is the result of incorrect repairs or use of products made
by Spencer Italia S.r.l. Repairs must necessarily be carried out by an authorized Spencer Italia service centre, which in using original spare
parts will provide a quality repair service in strict accordance with the technical specifications given by the manufacturer. Spencer Italia
S.r.l. disclaims any liability for any damage, direct or indirect, which is a result of improper use of spare parts and/or otherwise of any
repairs made by an entity other than the Spencer service centres authorized to repair or make substitutions on this product and parts
and/or otherwise of any repairs made by an entity other than the Spencer service centres authorized to do so; the warranty will also be
invalidated.
Use only original components, spare parts and or accessories, approved by Spencer Italia S.r.l., in order to carry out any operation
without causing any alteration or modification to the device.
All maintenance and revision must be recorded and documented with the corresponding report for technical assistance; documentation
shall be maintained for at least 10 years from the end of life and must be made available to the competent authorities and/or the
manufacturer if requested.
The cleaning schedule for reusable products must be performed in accordance with the directions provided by the manufacturer in the
user manual, in order to avoid the risk of cross-infection due to the presence of secretions and/or residuals.
The device and all its components, after washing, should be allowed to dry completely before storing.
Regulatory requirements
As a distributor or end user of products manufactured and/or marketed by Spencer Italia S.r.l., you are strictly required to have a basic
knowledge of any legal requirements applying to the devices contained in this supply that are in power in the final destination
Country (including laws and norms regarding technical specifications and/or safety requirements) of the goods and therefore you are
also strictly required to have the necessary knowledge to guarantee all aspects regarding the total conformity of the products to the
regulations in the relevant territory.
Promptly notify Spencer Italia S.r.l. (already during the first product enquiry) when requesting in details regarding any revisions to be
made by manufacturer in order to guarantee the conformity of the products to the territory’s legal specifications (including those
resulting from rules and/or norms of other kind).
Act, with all due care and diligence, and contribute to ensure conformity to general safety requirements of all devices marketed in the
territory, by providing final users with all necessary information for carrying out periodical checks on their devices, exactly as specified in
the relevant user’s manual.
Actively contribute to safety checks on product sold, by communicating any relevant risk analysis information both to the manufacturer
and to any competent authorities so that the necessary action can be promptly taken.
The distributor or final user is aware that in the event of any failure to conform to the above mentioned requirements you will be
deemed fully responsible for all damages that might occur. Therefore Spencer Italia S.r.l. expressly disclaims any responsibility and/or
liability for your non-compliance with the present regulatory provisions.
Avvertenze generali per dispositivi medici
L’utilizzatore deve leggere attentamente, in aggiunta alle avvertenze generali, anche quelle di seguito elencate.
It is not foreseen that the use of the device is prolonged beyond the time necessary for the first responders to the complete their
operation and the subsequent stages of transport to the nearest rescue point.
When the device is being used, the assistance of qualified staff must be guaranteed and at least one operator must be present.
Follow the procedures and protocols approved by the internal organization.
If disposable accessories are used, use only once and for only one patient. Do not wash or sterilize after use. Reuse may cause cross
infection. Some symbols contained in this manual refer to the standard accessories included In the purchased device.
The activities of disinfection and sterilization should be carried out in accordance with the parameters given in the validated cycle as
specified in the technical standards. Sterilization could reduce the lifespan of the devices.
Don’t use accessories after the expiration date indicated on the package, if present.
With reference to the D. Lgs. 24th February 1997, n. 46 emended by D. Lgs. 25/01/2010, n. 37 –Acknowledgement of Directive
93/42/CEE and 2007/47/CE concerning Medical Devices, we remind both public and private operators, , That in the exercise of their
activity detect an accident involving a medical product are required to notify the Ministry of Health, under the terms and in the manner
established by the relative ministerial decrees and also to the manufacturer. Health care providers whether public or private are