EME Shock Med User manual


Rev.3 –30/11/2020
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
I
IN
ND
DI
IC
CE
E
INFORMATION ON THE MANUAL ...........................................................................1
WRITING CONVENTIONS............................................................................................1
GARANZIA.............................................................................................................2
NOTES...................................................................................................................2
CAUTIONS .............................................................................................................3
! WARNING ! .........................................................................................................4
INTRODUCTION OF THE TECNOLOGY......................................................................5
Shockwaves................................................................................................................ 5
Propagation and diffusion speed of shockwaves.......................................................5
Generation systems of shockwaves...........................................................................5
The action mechanism...............................................................................................6
IN GENERAL...........................................................................................................6
INTENDED USE.......................................................................................................6
INDICATIONS ..............................................................................................................7
CONTRA-INDICATIONS ...............................................................................................8
PRELIMINARY NOTES.............................................................................................9
UNPACKING................................................................................................................9
SETTING UP ..............................................................................................................10
SEPARABLE COMPONENTS.......................................................................................10
CONNECTIONS..........................................................................................................10
DESCRIPTION OF THE EQUIPMENT .......................................................................11
REAR PANEL..............................................................................................................12
SEPARABLE COMPONENTS.......................................................................................12
UTILIZZO DELLA MACCHINA .................................................................................13
BEST USE ..................................................................................................................13
FREE PROCEDURE ................................................................................................14
MODIFY ....................................................................................................................14
SAVE .........................................................................................................................16
START .......................................................................................................................16
LOAD.........................................................................................................................18
PATHOLOGIES .....................................................................................................19
PATIENT FILES..................................................................................................... 20
SETTINGS............................................................................................................ 21
SETTINGS...................................................................................................................22
HISTORICAL...............................................................................................................22
DEVICE MAINTENANCE.............................................................................................22
EXECUTION OF THE TREATMENT ......................................................................... 22
MAINTENANCE ................................................................................................... 23
WORKING PROBLEMS ......................................................................................... 24
ELECTRO-MAGNETIC INTERFERENCES .................................................................. 25
TROUBLESHOOTING CHART................................................................................. 25
TECHNICAL FEATURES ......................................................................................... 26
APPENDICES ....................................................................................................... 27
Appendix A - ENVIRONMENTAL CONSIDERATIONS..................................................27
Appendice B –ETICHETTE.........................................................................................27
Appendice C –LIST OF THERAPEUTIC SUGGESTIONS...............................................28
Appendix D –REFERENCE TABLE FOR THE USE OF THE APPLICATOR ......................29
Appendice E –ELECTRO-MAGNETIC COMPATIBILITY TABLES..................................30
SHOCK-WAVE ACTUATOR REPLACEMENNT PROCEDURE ...................................... 32
PROCEDURE OF TRANSMITTERS REPLACEMENT................................................... 33

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
1
I
IN
NF
FO
OR
RM
MA
AT
TI
IO
ON
N
O
ON
N
T
TH
HE
E
M
MA
AN
NU
UA
AL
L
This manual is addressed to:
- user of the machine;
- owner;
- responsible;
- people in charge of moving;
- installers;
- users;
- people in charge of maintenance.
This document provides valuable information regarding the installation, set up and
use of SHOCK MED equipment.
It is a useful and essential reference guide for the user: read the contents of the
manual carefully before installing the equipment and keep it on hand at all times for
future reference.
It is of vital importance that you strictly adhere to the recommendations contained
within the manual in order to avoid malfunction, which may cause damage to the
equipment and consequent annulment of the validity of the warranty.
Furthermore, in order to obtain the highly efficient technical service available from
the manufacturer, it is essential that any handling of the equipment be in accordance
with the instructions provided.
The limits of this manual are:
- the user manual cannot replace proper experience;
- the user manual, for particularly difficult operations, can only be a reminder of the
main operations.
The manual is to be considered part of the equipment and must be preserved for
future reference until the decommissioning of equipment. The operating instructions
must be available for consultation in the vicinity of the machine and properly stored.
This manual reflects the state of the art at the time of sale and cannot be considered
inadequate if later updated based on new information. The manufacturer has the
right to update products and manuals without necessarily updating preceding
products or manuals.
The company will not assume any responsibility for any of the following major cases:
- improper use of the machine;
- use against the specific national regulations;
- incorrect installation;
- defects in power;
- serious shortcomings in maintenance;
- changes and unauthorized interventions;
- use of parts or materials not specific to the model;
- total or partial non-observance of the instructions;
- exceptional events.
If you would like any further information, please get directly in touch with the
company EME srl, to stay up to date on the best ways to use these machines and to
receive the necessary assistance.
W
WR
RI
IT
TI
IN
NG
G
C
CO
ON
NV
VE
EN
NT
TI
IO
ON
NS
S
Certain sections of the manual have been underlined in order to highlight their
importance.
N
NO
OT
TE
E
The notes contain important information and useful tips for operating the equipment.
C
CA
AU
UT
TI
IO
ON
NS
S
The CAUTION message appears before operations, which, if not correctly performed,
may cause damage to the machine and/or its separable components.
!
!
W
WA
AR
RN
NI
IN
NG
G
!
!
The warning message signals operations or situations, which, if unknown to the
operator, or incorrectly carried out, may harm the operator.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
2
G
GA
AR
RA
AN
NZ
ZI
IA
A
EME srl guarantees the quality of its products, when used in accordance with the
instructions provided in this manual, following these modalities:
-The device's warranty has duration for 24 months from the date of purchase.
Wear parts, which are not covered by the 24 month-standard warranty of the device,
are:
- APPLICATOR probe
- Transmitter/s
- INTERCHANGEABLE KIT
Note: as far as the INTERCHANGEABLE KIT is concerned, its warranty is valid 6 months
from the date of purchase, unless there are damages due to inappropriate use or
improper maintenance.
During the warranty period the company can provide for the replacement or
substitution of faulty parts
The warranty does not however, include the replacement of the equipment.
The warranty does not cover any malfunction or damage caused by:
1) incorrect connection and installation;
2) incorrect use due to non-compliance with instructions contained in this manual;
3) use of the machine in environmental conditions which do not conform with
those specified for the product;
4) improper or inadequate maintenance;
5) unauthorized opening of the outer casing;
6) tampering or unauthorized modifications;
7) use of non-original separable components.
EME srl registered offices provide the warranty.
Should you need to return the goods then please note the packing instructions as
follows. Enclose a copy of the purchasing receipt.
You should insure the postal package.
Before sending back the machine for suspected malfunction, we recommend that first
you carefully consult the sections regarding MAINTENANCE and TROUBLESHOOTING
of the manual, as a large part of the problems and faults are usually due to
inadequate maintenance or small technical problems which can often be easily solved
by the user himself.
A simple call to EME srl technical department may prove to be the solution to the
problem .
When re-packing the equipment for return to the manufacturer, proceed as follows:
1. unplug the machine and any connections, devices, applicators etc;
2. carefully clean and disinfect all parts of the machine and separable components
which have
been in contact with patients. Any equipment which the technical department
does not consider hygienic (Italian law T.U.S. 81/2008 on safety in the
workplace) will not be accepted;
3. disassemble separable components and any mechanical supports;
4. use original box and packing materials;
5. enclose detailed information regarding the nature of the problem in order to
facilitate the technical department’s intervention and save time on repair.
N
NO
OT
TE
ES
S
PRELIMINARY NOTES
−The installation of the device does not require any special care, is therefore simple and
immediate.
USE
−The keys shown on the display are touch in order to navigate the software.
−Shock Med devices recognize automatically the applicator plugged in to the output
connector.
−When using the function MODIFY PATIENT FILE, click on ENTER to store new data, by
doing so you shall cancel and overwrite any previous data. Old data shall then not be
retrievable.
−Parameter values modified during the treatment cannot be stored directly in the patient
file; therefore you shall have to create a personalised treatment, following the
instructions given in USER PROGRAMS.
−When using the function CREATE FILE it is mandatory that you fill in the fields NAME or
SURNAME and TREATMENT PROTOCOL of the disease. Failure enter does not allow saving
the patient card.
−Every time you click on the button START o STOP the device shall emit a long beep sound
as confirmation.
−After clicking on the button START, when setting off a treatment, the button shall then
read STOP and vice versa.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
3
−RESTORE FACTORY SETTINGS means deleting all tabs patients and custom protocols stored
in the user; they will no longer be recoverable.
−The files and programs deleted with the function DELETE shall not be retrievable.
−After starting the function SCREEN CALIBRATION you shall have to complete it, there is no
possibility to press the button ESC.
MAINTENANCE
−For an optimal use of the device and to guarantee its maximum performance, it is
recommended to perform maintenance at the correct time and in the suggested ways.
-The maintenance of the applicator kit, through the use of the brush supplied with the
equipment, allows to:
oClean the barrel from scroll residues of the bullet;
oLubricate the scroll barrel of the bullet to avoid frictions and air leaks
C
CA
AU
UT
TI
IO
ON
NS
S
PRELIMINARY NOTES
-The customer is liable for all damage caused by inadequate packaging of the material.
Keep the original packaging of the unit: it will be needed if the unit is returned to the
company.
-Do not use the equipment in places where it might get wet.
-Before operating the machine carefully check the correctness of the connections
according to the instructions.
-To avoid the risk of electric shock, this device must only be connected to power supply
networks with protective earth.
-Do not use separable components other than the ones provided: they might damage the
unit, causing the warranty to become void. In case you have any problems or difficulties
with installation, contact EME srl technical support.
If using the same extension for the unit and other units, make sure that the total current
being absorbed by the connected units, does not exceed the max current allowed for that
type of cable and that, however, it does not exceed 15 A.
-The therapeutic suggestions are stored in the permanent memory of the machine. These
protocols can be edited but it is not possible to save any changes.
-The protocols of therapeutic suggestion preloaded on the machine cannot be deleted.
-It is not possible to define a number of suggested sessions to evaluate the effectiveness
of the treatment, since they are related to the power delivered to the patient undergoing
treatment. It is a task of the physician to decide the number of therapy sessions which
subject the patient according to the specific requirements of the case, in order to ensure
to the patient himself the execution of an effective treatment in time and place in
conditions of absolute safety.
-Always control at times the integrity of the cable and of the applicator connector: they
must not be damaged or worn.
-It is a class B device in terms of emissions. The machine can be used in a hospital or
outpatient environment, as long as it is duly taken into account that the same machine
could disturb electronic devices placed in the immediate vicinity.
-Do not use the machine near HF SURGERY DEVICES and rooms with an RF shield of an EM
system for magnetic resonance, in which the intensity of the EM DISORDERS is high.
-No modification of this device is allowed.
-The use of separable components, transducers and cables, other than those specified or
supplied by EME srl, could lead to higher electromagnetic emissions or a decrease in the
level of electromagnetic immunity of the appliance, with consequent incorrect operation.
USE
-On request we can provide the user manual in electronic form.
-Because of security reasons, only the specific software must be loaded into each machine.
In case of exchange of software, the machine may immediately stop all its functions,
requiring the intervention of EME srl technical assistance.
-Use a different name for each personalised program. You can avoid inserting the same
name by checking in advance the list of the therapies stored.
-Before saving a personalised program check that you actually gave it a name, otherwise
the program will not have a reference name.
-We advice the operator to familiarize with the typical marked noises, related to frequency
and intensity of emitted pressure, that are connected to the emission of shockwaves.
-The appliance or the system must not be used near other equipment and, if it is necessary
to use it near other equipment, the medical electrical equipment must be observed to
check normal operation in the configuration in which it is used.
-If the electro-medical device, interacting with another device, causes or receives
detectable interferences, the user is invited to limit the interference by adopting one or
more of the following measures:
oReorient or reposition the receiving device;
oIncrease the distance between the devices;
oConnect the equipment to a scale of a circuit different from or to devices
that cause interference;
oContact the manufacturer or local technician for assistance.
-Portable and mobile radiocomunication devices can affect the operation of the device.
-transportable RF communication equipment (including peripherals such as antenna cables
and external antennas) should be used at a distance not less than 30 cm (12 inches) from
any part of the device, including the specified cables. Otherwise, the performance of this
device may be degraded.
MAINTENANCE
-Use the applicator with care: any misuse may affect their performance and features.
-Under no circumstances technicians not authorized by EME srl are allowed to open and/or
disassemble the applicator: such tampering, besides damaging its characteristics,
immediately invalidates the right to warranty.
-The equipment should never be disassembled for cleaning or inspection purposes: the
units does not have to be cleaned internally, and if for some reason the unit must be
opened, it should only be done by specialized technicians authorized by EME srl.
-Do not use thinners, detergents, acid solutions, aggressive solutions or flammable liquids
to clean the external parts of the unit and separable components. Using these substances,
or misusing the separable components, will cause the immediate voiding of all warranty
rights, as well as irreparably damaging the unit and the separable components.
-For optimal use of the apparatus and to ensure its optimum performances it is
recommended to perform properly within the time and in the manner recommended in
the maintenance actions.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
4
-For a correct replacement of the installed fuses, observe the following indications:
1. disconnect the power supply and open the fuse box using a screwdriver, makingsure
you insert the screwdriver in the slot on the fuse box and levering up outwards;
2. insert a screwdriver into the two side holes for fuse expulsion
3. remove the old fuses
4. insert a new fuse at a time by using a slight pressure to the left, with a finger
5. push the box back to fit into the slot.
−It is recommended to perform periodic maintenance every two years, in order to check:
othe intensity of any leakage currents;
othe continuity and thus the integrity, of the ground conductor;
othe correctness of the value of insulation resistance;
In order to ensure the electrical safety of the device, ensure that it is operating in a safe
guaranteed way. For this kind of intervention you should contact a qualified service
technician or alternatively EME srl or one of its authorized service centers.
-For the correct maintenance of the applicator kit, perform every two weeks the
procedure of cleaning through the supplied brush. Don’t completely insert the brush into
the barrel and never force the insertion
WORKING PROBLEM
-Only technicians authorized by the manufacturer may access the interior of the unit.
-You should contact EME srl or its authorized service centers for any repair work or further
information.
!
!
W
WA
AR
RN
NI
IN
NG
G
!
!
PRELIMINARY NOTES
-In order to avoid tipping over, the correct position of transport is such that the device
SHOCK MED is moved only along the direction x (both sides), holding it with both hands
on the curved profile of the device.
-The perfect functionality of the device is guaranteed in accordance with the rules of
installation and of use included, only with original separable components and spare parts.
-If there are problems or installation difficulties, please contact the EME srl technical
assistance department.
-Before connecting the cable to the mains plug, check that the equipment wasn’t damaged
during transport. Ensure that the power supply specifications on the mains socket
correspond with the information on the label attached to the back of the unit.
-The electric current that powers the unit is VERY DANGEROUS. Before connecting or
disconnecting the power cable from the connecter on the unit, make sure it is plugged out
from the mains socket.
-The power cable has an earthed plug for safety reasons.
-Only use with a mains socket suitable for use with earthed systems
-The equipment should only be connected to electrical systems that fully comply with
-regulations.
-If using extension cords verify the presence and the integrity of the protective conductor
to earth.
-Connect the equipment directly to the wall socket without using extensions. Failure to
comply with these warnings may result in dangerous electrical discharges that could cause
injury operators and compromise the functioning of the unit.
-The manufacturer is held responsible for the fundamental safety, reliability and
performance of the device only if:
oThe electrical system of the premises complies with the appropriate regulations;
oThe device is used in accordance with the instructions for use.
USE
-Shock wave therapy treatments must be provided, under the strict control of the
operator, to "conscious" patients, able to interact with the operator in the face of the
stresses transmitted by the machine.
-If the pain threshold of the patient does not allow the emission of the expected maximum
energy density, it is advisable to use the maximum tolerated level. To achieve the
maximum expected or tolerated energy in protocol you have to increase the energy
density each 100 pulses.
-Before switching on the generator, adjust the nut on the correct value of the main voltage
in use in the room where the treatment is carried out to avoid malfunction of the
machine.
-DO NOT EVER START THE APPLICATOR BEFORE YOU HAVE CORRECTLY INSERTED THE
SUPPLIED HEAD. DAMAGES OF THE SUPPLIED GUN IN THESE CONDITIONS ARE NOT
COVERED BY WARRANTY.
-To get a perfect recognition of the applicator connected to the output channel, it is
strongly recommended to connect / disconnect them when the emission of treatment is
interrupted.
-Once you have started a program, the buttons on the toolbar are disabled; the only
allowed operation is stopping the program by pressing the button STOP.
-In order to guarantee that the device works in safety conditions for the client, we advise
that the device undergoes periodical check tests (at least every 2 years), as the device
contains parts subject to electrical degradation and aging, such as the compressor.
-It is strictly forbidden to use the device in environments with high oxygen levels. If you fail
to abide by this rule, EME srl shall not be deemed responsible for any accidents.
-It is absolutely forbidden to cover the ventilation slots: such an action may not allow the
machine to work in safe conditions. In case of non-compliance with this indication, EME srl
will not be responsible for any accidents.
-It is important to pay the attention of the operator to the necessity to verify the
correctness of the electric installation of device before activating the supply switch.
-It is advisable to suspend the therapeutic treatment if there were to appear some
disturbances during its emission.
-Before starting a treatment clean thoroughly and disinfect all the separable components
and the parts of the device that got in contact with the patient, in particular the
shockwave transmitters.
MAINTENANCE
-It is forbidden to remove the electrical/pneumatic connector of the applicator without
first having downloaded the pneumatic circuit. So TURN OFF your device with the main
switch and wait 10 seconds for pneumatic discharge. This procedure is introduced to
safeguard the integrity of the O-Ring inserted into the connector.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
5
-For safety reasons before carrying out any maintenance or cleaning of the unit, YOU
MUST turn off the equipment with the power switch at the back and unplug the socket
connected to the mains.
-Before every treatment, it is recommended to clean with caution all of the separable
components and the parts of the equipment that have been in contact with the patient.
-The cleaning and disinfection must be done systematically before the therapeutic
treatment conducted on the patient.
-We advise the operator to carry out periodical maintenance of probes/applicators, in
particular:
➢Check the head of the applicator to make sure there are no cracks, wherefrom
conductive liquid may penetrate;
➢Check the integrity of the cable and the connector of the probe/applicator.
-Do not spray or pour liquids on the external case of the device, air slots, the LCD touch-
screen display or near it. In you fail to do so, inspect the device. EME srl will not be
responsible for any damages on the device, if the abovementioned conditions will not be
respected.
-Always control at times the integrity of the cable and of the applicator connector: they
must not be damaged or worn.
-It is advised that personnel with technical preparation substitute the fuses, to perform the
operation in safety conditions.
-Do not open the device: inside there are high voltages that may be hazardous.
-Only personnel authorized by the manufacturer may access the internal components. For
repairs and further information please contact EME srl or its authorized service centers.
WORKING PROBLEM
−DO NOT OPEN the unit, as HIGH VOLTAGE ELECTRICITY is present and may prove VERY
DANGEROUS.
I
IN
NT
TR
RO
OD
DU
UC
CT
TI
IO
ON
N
O
OF
F
T
TH
HE
E
T
TE
EC
CN
NO
OL
LO
OG
GY
Y
S
Sh
ho
oc
ck
kw
wa
av
ve
es
s
From a physical point of view shockwaves are defined as high-energy acoustic waves.
Specifically, they are pressure pulses generating direct mechanical force with the aim
to convey energy to body tissues in order to stimulate healing processes.
Shockwave should not be confused with ultrasound wave that is normally used for
diagnostic and therapeutic purposes. Unlike ultrasound, shockwave has a pulsed form
and provide much higher pressure values, on average 1000 times higher.
The shockwaves used in therapy are specific acoustic waves with characteristics
defined at the international level (D.I.G.E.S.T). In order to help the users make
reliable assessments for the therapy and the research, the most representative
parameters have been chosen and agreed upon by the International Society for
Medical Shockwave Therapy (ISMST) and the producers of shockwave devices:
-
-
the pressure (measured in MPa, 1MPa=10 bar that is about 10 atmospheres):
SHOCK MED can generate up to 5bar of pressure and SHOCK MED SP up to 4 bar
pressure;
-
-
the energy flux density (measured in mJ/mm2);
-
-
the energy (measured in mJ);
-
-
the dimensions of the focal volume, determined conventionally at 50% of the
maximum pressure.
P
Pr
ro
op
pa
ag
ga
at
ti
io
on
n
a
an
nd
d
d
di
if
ff
fu
us
si
io
on
n
s
sp
pe
ee
ed
d
o
of
f
s
sh
ho
oc
ck
kw
wa
av
ve
es
s
Shockwave propagation speed, as with each acoustic wave, is particularly related to
the means that conveys it and to the intensity of the shockwave.
Biological structures like cell walls, whose thickness is comparable to few molecular
layers, undergo very high pressure gradients when shockwaves pass.
The mechanical properties of the biological means that are hit by the shockwave, like
elastic and compressible properties, influence the transmission of shockwave
determining the propagation speed.
When shockwaves pass through a fluid, they generate various pressure differences
that produce gas bubbles. The next shockwave hitting these bubble generate a strong
implosion that will produce liquid spilling all over the tissue to be treated. As a
consequence to these lesions some biological events occur, they differ according to
the hit tissue.
In specific, in the bone tissue osteogenic and vascular reactions were observed, while
in soft tissues there were anti-inflammatory, antalgic and vascular reactions.
The spreading of the acoustic wave in the tissues follows the laws of physics of
acoustic wave that concern transmission, reflexion and absorption. These laws are
linked to the characteristics of the means and are influenced inevitably by differing
values of density and impedance of the skin, fat, muscles and bones.
G
Ge
en
ne
er
ra
at
ti
io
on
n
s
sy
ys
st
te
em
ms
s
o
of
f
s
sh
ho
oc
ck
kw
wa
av
ve
es
s
There are different types of equipment for shockwave therapy that differ in the
technology used to generate the waves. Generally speaking a shockwave generator is
composed of:
- a device that creates the pressure shot;
- a water chamber for the concentration of the shockwave energy in the desired focal
volume or a dome-shaped gum membrane to close the output gate of the
shockwaves.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
6
The membrane functions as a coupling means with the skin of the patient, or a
ballistic system composed of a spring-geared metallic applicator (radial shockwaves).
In the medical field shockwave are created by a strong immediate increase of
pressure inside the water chamber or the ballistic system.
In specific, in a ballistic or radial system the shockwave is generated in a gun-shaped
applicator, where one end is closed by a metallic “lid” against which a steal gun is
shot by means of compressed air at 4-5 bars. Following the collision the shockwave is
generated and, through the metallic lid, it spreads radially in the skin and the first
layer behind the skin.
T
Th
he
e
a
ac
ct
ti
io
on
n
m
me
ec
ch
ha
an
ni
is
sm
m
The action mechanism in the muscle-skeletal tissues is very complex and it is still
subject to an in-depth research. The shockwaves act in different ways according to
the different pathologic tissue they treat (bone, soft tissues, skin). Generally speaking,
they stimulate the activation of natural biologic repair processes.
The action mechanism of the shockwaves seems to originate in two main effects:
1. direct physic-mechanical effects: the so-called “cavitation effect” and micro-
streaming with consequent creation of new blood vessels that increases local afflux
of blood and the production of new cells that speeds up the reparation of micro-
lesions and improves tissue trophism;
2. indirect biological effects induce:
the reduction of the pain transmission stimulating nerve-ends and freeing substances
that regulate its perception; vascularisation that produces bio-molecular
modifications.
I
IN
N
G
GE
EN
NE
ER
RA
AL
L
EME srl has recently developed a complete series of apparatus, accessories and
equipment, designed and manufactured according to the highest standards of quality,
making use of the latest technology and fully adhering to current directives and
norms.
Particular attention has been paid to the design, easy operation, function and safety
of the equipment and the final result is this modern, compact unit, which offers an
extremely logical operative sequence supported by a clearly legible display .
A wide range of therapeutic applications, and guaranteed patient and therapist safety
ensure that equipment is of the highest quality.
The equipment was planned and built in a manner that their use, if it happens at the
conditions indicated, does not compromise the health and safety of the patients, of
the users and of third, taking into consideration the benefit to the patient.
Such equipment is not bound to diagnosis, prevention, monitoring, compensation of
injury or handicap, substitution or modification of the anatomy, control of the
conception, support/vital support of functions but allow to treat special pathologies
and to reduce the illness.
A special intervention is not required in the event of failure of the medical device, but
just a normal maintenance/repair.
I
IN
NT
TE
EN
ND
DE
ED
D
U
US
SE
E
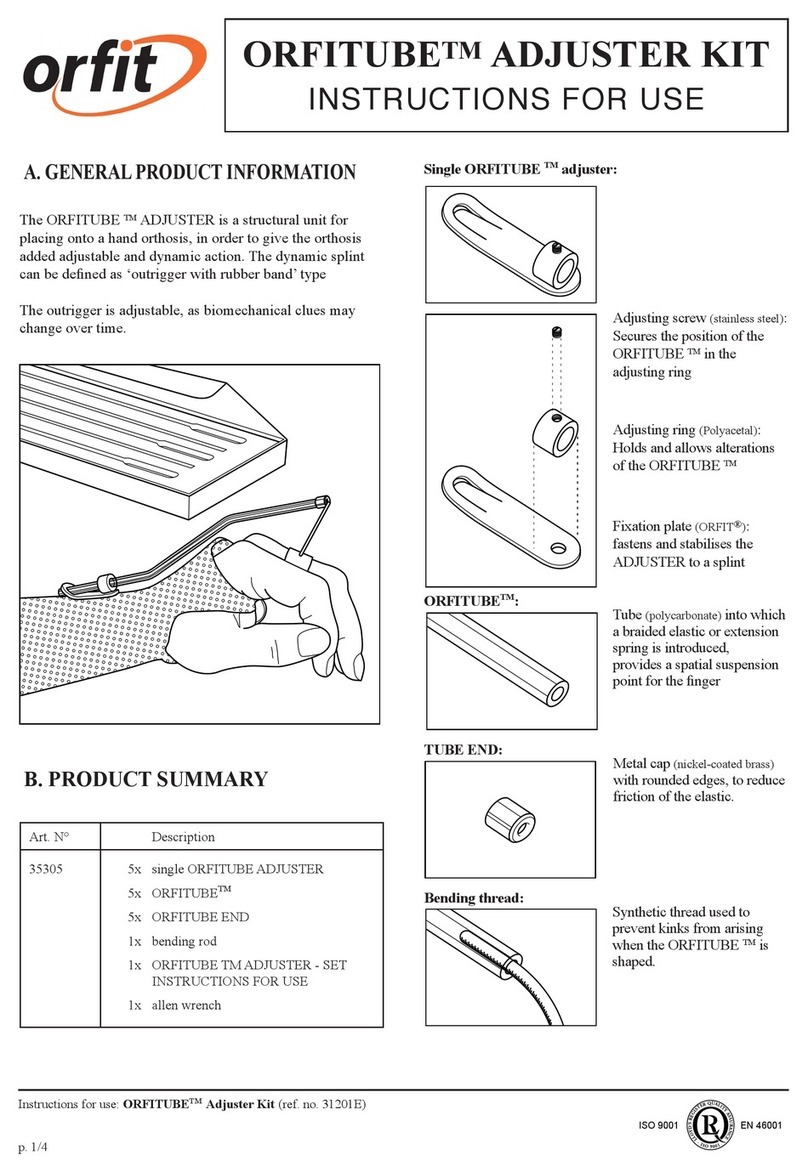
SHOCK MED e SHOCK MED SP are an electro-medical device that provide therapeutic
treatment that uses shock waves through the aid of a special applicator.
Shock waves are acoustic waves that carry high energy, transmitted through the
surface of the skin and spread radially in the body, in the area of pain. The body
responds with increased metabolic activity in the area of application, helping to
decrease the inflammation caused by analgesic action induced by the local release of
endorphins, stimulating and accelerating the healing process.
The use of this equipment SHOCK MED is reserved for operators such as physiatrists,
physiotherapists and pain therapists that, by their training, provide assurance of
proper use and safety for the patient.
In fact, the operator must be appropriately qualified and he carefully studied the
contents of the user manual in order to use the device; or, he must operate under the
supervision of a health professional adequately qualified to use the machine, able to
understand the benefits and the limits of therapy and to work in conditions of safety
for the person undergoing treatment.
Such equipment can be used in hospital environment and on an outpatient basis.
Nevertheless, it is important to know that the user follow the medical instructions to
use the equipment or that he follows the indications present in the user’s manual.
SHOCK MED are radial shock waves, since the shock wave is generated by means of a
special shaped handpiece gun, whose barrel is closed at the end by a metal cap
against which a projectile of steel is launched by means of compressed air (5bar
pressure).

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
7
This generates a shock wave which spreads radially expanding in the skin and
underlying first layer of tissue, or in a focused way (depending on the transmitter
used). The measure of the penetration depth varies from 4 to 7 cm.
I
IN
ND
DI
IC
CA
AT
TI
IO
ON
NS
S
The main applications are used in the following fields: Orthopedics, Rehabilitation and
Sports Medicine.
The shock wave method is the preferred treatment for chronic insertional
tendinopathy, characterized by poor vascularization osteotendinea junction, where
the physiotherapy treatment (infiltration and laser therapy) has proven ineffective.
Here are the main diseases on which the shock waves are applied:
Elbow: epicondylitis and epitrocleitis
Epicondylitis and epitrocleitis are two inflammatory diseases due to a degeneration of
the tendon insertion of the epicondylar muscles, that is the extensor muscles, and of
the epitrochlear muscles, that is, the flexor muscles, of the elbow.
These pathologies arise as a consequence of tendon overload due to a continuous
stress of the insertion of the aforementioned muscles.
Lateral epicondylitis, also known as "tennis elbow", is a syndrome that occurs in
subjects who repeatedly perform pronation and supination movements of the
forearm in a condition of complete elbow extension. It manifests itself as lateral pain
in the elbow, during the extension of the wrist and high intensity pain during the
movement performed to grasp objects.
Even in the case of epithrocleitis, or "golfer's elbow", tendon degeneration occurs
due to incorrect use of the joint of its tendons.
The effectiveness of the shock wave treatment for the indicated pathologies seems to
be due to the neovascularization of the tendon-bone junctions; in fact, by improving
the flow of blood into the tissues, an increase in cell proliferation is achieved, which
leads to the regeneration of tendon and bone tissues.
Shoulder: insertional tendinopathies, impingement, myofascial pain
TENDONS are robust fibrous structures, with a pearly complexion, which unite
muscles to bones. These important anatomical structures therefore function as real
connections, capable of transforming the force generated by muscle contraction into
movement.
Like all anatomical structures, tendons can also undergo degenerative phenomena
with the passage of time. Furthermore, tendons have long healing times and a
marked propensity to evolve into a state of chronic inflammation.
The term tendinopathy generically refers to a painful condition that develops in or
around the tendon when subjected to overuse. When this involves the shoulder, we
speak of "insertional tendinopathy of the rotator cuff", that is, inflammation of the
tendons of some of the muscles responsible for shoulder movement, such as the
supraspinatus, infraspinatus and teres minor.
The most common tendinopathies are those affecting the supraspinatus and
infraspinatus, less common are those involving the subscapularis.
Impingement syndrome is a disease that can lead to the gradual degeneration of the
supraspinatus muscle tendon. In impingement syndrome, during the lifting
movement of the arm and in the phase of returning to the initial position, a
compression of the tendon of the supraspinatus muscle occurs, which generates pain.
The narrowing of the subacromial space due to anatomical causes or biomechanical
alterations of the shoulder (e.g. imbalance between the rotator cuff muscles, misuse
of the shoulder, chronic tension, repeated microtraumatism, etc.).
Impingement syndrome can lead to gradual degeneration of the tendons and, over
time, even rupture.
Another painful pathology that can affect the shoulder, in particular the trapezius
muscle, is myofascial pain syndrome (MPS). It is known to be a clinically common
syndrome with features including localized muscle pain, palpable intramuscular tight
band, referred pain, and muscle spasm following trigger point stresses. MPS is
frequently encountered in clinical settings and represents the majority of
musculoskeletal diseases. The primary goals of treatment are the inactivation of the
trigger point, the relaxation of localized contracture and the interruption of the
vicious pain-spasm-pain cycle.
Knee: patellar or goose leg tendinopathies, osteoarthritis
The patellar tendon connects the lower part of the patella with the upper part of the
tibia and its function is to transmit the contraction of the quadriceps muscle to the
tibia to extend the leg.
Patellar tendinopathy is a knee disorder that affects the part of the tendon below the
patella. In most cases, the resulting pain is caused by chronic and continuous stress
on the patellar tendon leading to small lesions, which can degenerate over time.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
8
Patellar tendinopathy is a very frequent pathology and the subjects most at risk are
those who practice sports in which the ischio-perineo-tibial muscles are subjected to
continuous stress.
Another very common painful pathology, which does not involve the tendons of the
knee joint is osteoarthritis. It is a chronic inflammation of the knee caused by the
degeneration of the articular cartilage. In the absence of joint cartilage, the femur
and tibia rub together causing pain. The causes of knee osteoarthritis are various and
can be traced back to various factors such as age, weight or traumatic events.
Pube: tendinopatie degli adduttori (pubalgie)
The adductor muscles are large muscles that allow a limb to be brought closer to the
median axis of the body.
Adductor tendinopathy, also known as "hip adductor syndrome" or simply "groin",
particularly affects the insertion of the adductor longus pubis and the pectineus
muscle. It can be caused by repeated microtrauma or following an episode of muscle
distraction that has not been properly treated.
Adductor insertional tendinopathy is typical in people who play sports that require a
high frequency of explosive actions and is generally caused by an imprudent or
incomplete preparation of the athlete.
In initial cases, the pain appears upon awakening and at the beginning of sporting
activity and then disappears once the athlete has warmed up. In the most severe
forms, the pain does not subside as a result of muscle heating but tends to worsen to
the point of compromising the continuation of activity.
Ankle: Achilles tendinopathies, calcaneal apophysites.
The Achilles tendon is the largest tendon in the human body, capable of withstanding
a load capacity of up to about 12.5 times the body weight and which connects the calf
muscles to the heel.
Achilles tendinopathy involves inflammation of the Achilles tendon and is generally
caused by an injury that occurred during running or sports.
An apophysitis is an inflammatory pathological state of an apophysis, that is, a bony
prominence. Calcaneal apophysitis, or Sever's disease, is an inflammation of the
apophysis of the calcaneus, where the Achilles tendon is inserted. It mostly appears
following a sudden increase in workloads in children between the ages of 9 and 15.
The cause for this pathology seems to be the tension exerted by the Achilles tendon
on the calcaneal tuberosity which, not yet fully ossified during adolescence, is pulled
away from the calcification core, inflaming the growth plate. A contributing factor
may be the repeated stress caused by the impact of the heel on the ground during
running and jumping.
Lower and upper limbs: muscle spasticity
Muscle spasticity is a common and disabling problem seen in approximately 50% of
stroke patients. Muscle spasticity was defined as a motor disorder characterized by
an excessive increase in muscle tone due to the hyper-excitability of the stretch
reflex. Alterations in neural networks as well as abnormalities in the mechanical
properties of muscles have been suggested as possible mechanisms underlying this
disorder.
Severe spasticity must be managed as it can cause long-term secondary complications
such as pain, soft tissue contractures, functional limitations, and reduced quality of
life.
Wrist: Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a clinical syndrome caused by compression of the
median nerve in the wrist. It is the most common entrapment neuropathy in adults.
Clinical features of CTS include night pain, numbness, tingling sensation in the median
nerve dermatome, and the diagnosis is confirmed by these typical clinical symptoms,
along with electrodiagnostic studies.
Low back pain
Low back pain is a painful manifestation localized in the lumbar region, generally
spread towards the buttocks, on one or both sides, which affects people of both sexes
in all periods of life.
Low back pain is defined as chronic when it lasts for at least 3-6 months without
interruption.
C
CO
ON
NT
TR
RA
A-
-I
IN
ND
DI
IC
CA
AT
TI
IO
ON
NS
S
Absolutes
-Pregnancy
-Coagulation disorders
-Presence of neoplasms of growth of nuclei in the field of applications
-Demyelinating polyneuropathy
-Infectious tenosynovitis
-The proximity of the lung parenchyma to the scope of application

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
9
-Acute infection of soft tissue/bone
-Epiphysiolysis in the focal point
-Patients with active implantable devices
-Brain, spinal cord, large nerves in the focal point (neurocranium, spinal
column, ribs)
-Severe osteoporosis. It should be noted that in case of severe osteoporosis,
i.e. when the T-score is greater than 3, of tendon injury, or of necrosis
advanced bone, it is not possible to perform a therapy with shock waves.
-Metal implants
-Use of vaso-constrictors medicines
Relatives
-Tear of the rotator cuff
-Tendinopathies associated with severe glenohumeral arthritis secondary to
instability or capsular ligament
-Primary pernicious diseases
-Epiphysiolysis in the focal point
-Diseases of blood coagulation and use of anti-coagulants
-Lung tissue in the focal point
Collateral effects:
-Hematomas and/or petechiae particularly with high-energy pulses
(>0,60mJ/mmq) ;
-Edema
-Exacerbation of symptoms over the next 2-3 days which either disappear by
themselves or with cryotherapy and analgesic.
-
P
PR
RE
EL
LI
IM
MI
IN
NA
AR
RY
Y
N
NO
OT
TE
ES
S
U
UN
NP
PA
AC
CK
KI
IN
NG
G
The equipment SHOCK MED is specially packaged for transport in a single pack with
filling which has been specifically studied for safe transportation and storage.
To remove the equipment from the pack, place the box on a smooth, flat surface.
Open the top of the box and remove the polystyrene filling. Be very careful when
removing the contents of the pack.
The unit and separable components are wrapped in transparent sheets of
polyethylene protection and contain the following:
- n. 1 User Manual;
- n. 1 mains power supply cable;
- n. 2 spare fuses (see technical specifications);
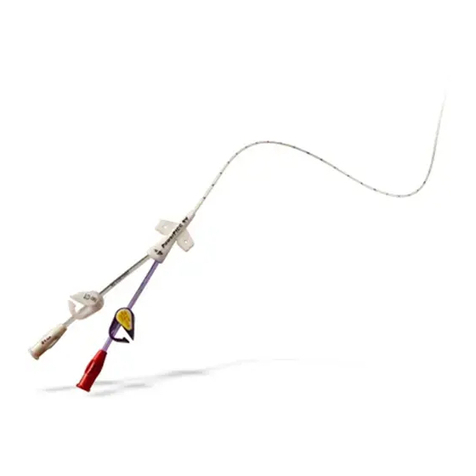
- n.1 SWT Shock-Med applicator probe;
- n.2 interchangeable KIT for the applicator(one inside the gun);
- n.1 multi-focused transmitter 9 mm;
- n.1 focused transmitter 15 mm;
- n.1 multi-focused transmitter 15 mm;
- n.1 lubricate brush;
- n.1 conductive gel pack 1000 ml;
- n.1 key tube removing actuator
- n.1 key blade removing actuator
- n.1 ring nut fixing knob
Check the contents of the package and should any of the items be missing then
contact your local authorized EME srl dealer.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
10
S
SE
ET
TT
TI
IN
NG
G
U
UP
P
Installation of the shockwave therapy equipment is fast and simple.
After placing the device, block the wheels with brakes in order to avoid undesired
movements.
The following environmental conditions are ideal when installing the equipment:
−room temperature: from +10° to +35°C;
−humidity level: from 10% to 80% without condensation;
−avoid direct exposure to sunlight, chemical products and vibrations;
−avoid using RF wireless communication devices in proximity (<0.30m).
S
SE
EP
PA
AR
RA
AB
BL
LE
E
C
CO
OM
MP
PO
ON
NE
EN
NT
TS
S
The devices can be used with the following separable components:
Description
Supplied
Optional
Power cable supply
1
Spare FUSES (see technical specifications)
1
Lubricate brush
1
User manual
1
SWT applicator
1
Bottle of gel 1000 ml
1
Focused transimitter 15 mm
1
Multi-focused transimitter 9 mm
1
Multi-focused transimitter 15 mm
1
Interchangeable KIT for the applicator
2 (one inside
the gun)
Key tube removing actuator
1
Key blade removing actuator
1
ring nut fixing knob
1
Suitcase for applicator + shaped foam
1
Applicator complete of transmitter 15 mm
X
Focused transimitter 15 mm
X
Multi-focused transimitter 9 mm
X
Multi-focused transimitter 15 mm
X
Multi-focused transimitter 35 mm
X
Wrench for 35mm focused transmitter
X
Interchangeable KIT for the applicator
X
Description
Supplied
Optional
American power cable supply
X
English power cable supply
X
The separable components that can be replaced by the RESPONSIBLE ORGANIZATION
and that can influence the conformity of the EM EQUIPMENT:
Pneumatic-electric hybrid cable: 1 pneumatic tube and 3 electric cables. The cable
length must be less than 3m.
The connection of the shockwave applicator is simple: we need to plug its connector
into the socket on the back of the machine.
Contact authorized dealers EME srl for problems or difficulty of installation.
We recommend using the Gel marketed by Fiab, model G009, or an equivalent gel.
C
CO
ON
NN
NE
EC
CT
TI
IO
ON
NS
S
The connection of the applicator is simple: you need to connect the cable to the
device, inserting it into the connector on the anterior panel.
The power entry module can be found on the back of the unit and consists of a three-
pole socket for the cable set, an extractible fuse box with two fuses (see technical
specifications) and the main switch.
Plug the three-pin plug of the power supply cable into the integrated board and
ensure that it is correctly plugged into the connector.
Once you have checked that installation and assembly have been carried out
according to instructions provided up to this point in the manual, switch on the
machine making sure that the display screen is turned on correctly.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
11
D
DE
ES
SC
CR
RI
IP
PT
TI
IO
ON
N
O
OF
F
T
TH
HE
E
E
EQ
QU
UI
IP
PM
ME
EN
NT
T
TOUCH SCREEN color
display
APPLICATOR
HANDPIECE
Front panel
Rear panel

SW2050
S
SH
HO
OC
CK
K
M
ME
ED
D
FT05MI11
12
F
FR
RO
ON
NT
T
P
PA
AN
NE
EL
L
R
RE
EA
AR
R
P
PA
AN
NE
EL
L
S
SE
EP
PA
AR
RA
AB
BL
LE
E
C
CO
OM
MP
PO
ON
NE
EN
NT
TS
S
General ON
/ OFF switch
Tripolar jack for
power cable
supply
Transmitter
APPLICATOR
TRANSMITTER
Fuses box
Actuator of
shockwave
Actuator removal
key
Knob key for
transmitters
Connection connector for
the APPLICATOR
handpiece
TUBE HANDLE including
actuator
Metal ring for the
tightening
Multi-focused
transmitter, 15
mm
Focused
transmitter, 15
mm
Multifocused
transmitter, 9
mm
Cart power cord socket (not in
use)
USB connector, cart data
connection (not in use)
Connector for
connecting the USB key
Lubricate Brush
Footswitch connector
(not in use)

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
13
U
UT
TI
IL
LI
IZ
ZZ
ZO
O
D
DE
EL
LL
LA
A
M
MA
AC
CC
CH
HI
IN
NA
A
This chapter will provide important information on the correct use of the SHOCK MED
shock wave therapy device.
All the control functions and the entire functional structure of the machine are
managed and coordinated by a microcontroller: in addition to the task of making the
application programs already stored available, it allows an optimal and safe use of the
device in a personalized way.
The dialogue interface with the user is carried out by a large graphic backlit liquid
crystal display (LCD) TOUCH SCREEN: all the operating messages of interest to the
operator are displayed on it, as well as the functional status of the machine during
normal operation. therapeutic activity, any error messages.
The following paragraphs illustrate the operations that must be carried out by the
operator to make the most of the potential and technical peculiarities of the SHOCK
MED device.
The different options are covered, from the selection of a pre-stored program for
setting up a specific therapy, to the determination of the correct working parameters
for a "customized" application.
The shock wave acoustic radiation delivered by SHOCK MED has a health purpose, so
it cannot be minimized.
Therefore, no means of protection are required in this sense for the patient, who
receives treatment for health purposes, nor for the operator, who is in no way
affected by the acoustic radiation emitted by the applicator handpiece.
F
FU
UN
NC
CT
TI
IO
ON
NI
IN
NG
G
The devices for shockwave therapy SHOCK MED have a control console optimized
depending on the specific field of use and type of operation for which they are
intended.
All operating parameters are managed and controlled in real time by a sophisticated
electronic circuit with microcontroller, with clear representation and signaling of the
various functions by means of a backlit LCD touch-screen display (located on the
machine) and appropriate acoustic signals.
SHOCK MED gives the possibility to save personalized programs and patient cards in
the memory support called USER MEMORY in which both personalized protocols and
patient files can be stored.
The standard therapeutic suggestion protocols are saved in an additional fixed
internal memory of the machine. This memory is not manageable by the user: the
data can neither be deleted nor formatted. To make any changes made available,
they must be stored on one of the alternative media, creating a customized protocol.
B
BE
ES
ST
T
U
US
SE
E
After installing and positioning the machine according to the instructions provided in
the previous chapters, and having applied the cable for connecting the handpiece to
the appropriate connector, insert the power plug into the wall socket (230Vac) and
activate the device setting the main ON / OFF switch on the rear panel to the “ON”
position.
This operation prepares SHOCK MED for operation, causing the lighting of the backlit
LCD display which signals the condition of the device ready to operate.
fig. 1
The LCD display will light up highlighting a presentation screen (fig. 1) and then a
PASSWORD ENTERING screen:
1. type the access PASSWORD
oin the event of an incorrect password, an information appears
that warns the user to type the password again
2. once the correct password has been entered, the main screen will be
accessed where it will be possible to select the desired operating mode from
the 4 available.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
14
The password has been set by default to 12345: to enter it, simply press the 5
numeric buttons in sequence and then the OK button. Entering the code prepares
SHOCK MED for operation.
This code can be modified by the user (see SETTINGS - DEVICE MAINTENANCE -
GENERAL section).
fig. 2
In the initial screen it is possible (Fig. 2):
-access the FREE PROCEDURE section
-access the PATHOLOGIES section
-access the PATIENT CARDS section
-access the SETTINGS section by clicking on the button at the bottom right.
The operation of each key will be described below.
Before starting any treatment it is very important to connect the handpiece to the
appropriate connector on the front panel of the machine.
F
FR
RE
EE
E
P
PR
RO
OC
CE
ED
DU
UR
RE
E
By pressing the FREE PROCEDURES button a screen appears (fig. 3) in which you can:
-modify the processing data, proceeding as indicated in MODIFY;
-save any modified parameters proceeding as indicated in SAVE;
-upload a personalized treatment as indicated in LOAD;
-start the treatment, following the START procedure.
fig3
M
MO
OD
DI
IF
FY
Y
In this section it is possible to modify the treatment parameter values set by default
in the machine in order to create customized programs.
1. Click on the parameter to be modified, the modification screen appears
showing the name of the parameter to be modified and it is possible to
increase or decrease the value using the + or - buttons or by scrolling the
cursor to the right or left until to reach the desired value;

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
15
fig 4
2. For the SHOCKS parameter (number), increase or decrease the value using
the + or - buttons or by scrolling the cursor to the right or left until the
desired value is reached (figure 4). It is possible to vary the URTI parameter
between 10 and 10000.
oClick on CONFIRM (green tick) to save the parameter set value and
return to the main screen;
oClick BACK (grey x) to cancel the parameter modification operation,
you return to the main screen without having made any changes.
fig.5
3. For the FREQUENCY (Hz) parameter, increase or decrease the value using the
+ or - buttons or by scrolling the cursor to the right or left until the desired
value is reached (figure 5). It is possible to vary the FREQUENCY parameter
between 1 and 20 Hz.
oClick on CONFIRM (green check) to save the parameter set value and
return to the main screen;
oClick BACK (grey x) to cancel the parameter modification operation,
you return to the main screen without having made any changes.
4. For the MODE parameter, change the mode using the + or - buttons until you
reach the desired one. This parameter represents the delivery mode of the
strokes emitted by the applicator handpiece during treatment, the choice is
between 5 emission modes: SINGLE, CONTINUE, BURST, CONTINUE AUTO,
BURST AUTO.
fig 6
-By selecting the BURST AUTO and BURST delivery modes, other
modifiable parameters appear on the screen (figure 6): SHOCKS or the
number of pulses that make up the burst (or pulse train) and PAUSE (ms)
(only in BURST AUTO mode) or the pause between consecutive bursts.
-By selecting the SINGLE delivery mode, the FREQUENCY parameter
cannot be changed.
oClick on CONFIRM (green check) to save the parameter set value and
return to the main screen;
oClick BACK (grey x) to cancel the parameter modification operation,
you return to the main screen without having made any changes.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
16
fig 7
5. For the INTENSITY (Bar) parameter, increase or decrease the value using the
+ or - buttons or by scrolling the cursor to the right or left until the desired
value is reached (figure 7). It is possible to vary the INTENSITY parameter
between 1.0 and 5.0.
oClick on CONFIRM (green check) to save the parameter set value and
return to the main screen;
oClick BACK (grey x) to cancel the parameter modification operation,
you return to the main screen without having made any changes.
6. For the DURATION (minutes) parameter it is a parameter that cannot be
changed directly but varies automatically as the number of SHOCKs, the
frequency and the MODE set vary.
S
SA
AV
VE
E
To save any changes made to the parameters and store a personalized therapy
program:
1. Click the SAVE button;
NB: It is possible to save the protocols only in the INTERNAL MEMORY of the
machine. It is not possible to store personalized treatments on the USB.
2. Type on the virtual keyboard the name to be assigned to the created therapy
program;
3. Click on CONFIRM (green tick) to continue with the program saving
operation;
oOtherwise, click on BACK (grey x) to cancel saving the therapy
program, the screen with the modified treatment;
4. To start the saved customized program proceed as described in the START
section.
When saving a new customized program, the software checks the programs already
present in the database.
If the therapeutic program has an existing identification name, the inability to save
data with that specific name will be reported unless you choose to overwrite the
therapy:
oClick YES to proceed with overwriting the therapy;
oClick NO to cancel therapy overwriting and enter a new name to be assigned
to the created therapy program.
S
ST
TA
AR
RT
T
By clicking START it will be possible to start the treatment according to the selected
delivery mode.
Connect the applicator handpiece in the appropriate connector on the front panel of
the machine.
-If you start the treatment without connecting the handpiece, an error
message "HANDPIECE ERROR" appears on the screen which prevents the
start of treatment.
BURST AUTO delivery METHOD
To start the provision of a treatment:
1. Place the applicator handpiece on the part to be treated;
2. proceed with the emission by initially pressing the trigger on the handpiece
(or the footswitch): in this way the emission is enabled autonomously;
3. At the end of the burst, the next train of pulses is automatically emitted after
a pause time set directly by the operator;
4. select STOP to end the treatment early,
5. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
17
CONTINUOUS AUTO delivery METHOD
To start the treatment:
1. place the handpiece on the part to be treated
2. touch the START button
3. proceed with the emission by initially pressing the trigger on the handpiece
(or the footswitch): this enables the autonomous emission of a succession of
impulses set for the delivery of the treatment.
4. to suspend dispensing, press the applicator trigger (or the footswitch
5. to resume the treatment, press the applicator trigger again (or the
footswitch);
6. select STOP to prematurely end the treatment,
7. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
BURST delivery method
To start the treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
3. proceed with the emission by pressing while continuously pressing the trigger
(or the footswitch) on the handpiece: this enables the emission of the first
pulse train (burst);
4. At the end of the burst, the next pulse train is automatically emitted after a
pause time set directly by the operator;
5. To suspend the delivery between one burst and another, remove the
pressure on the applicator trigger (or on the footswitch);
6. to resume the treatment, keep the trigger pressed continuously, as
previously seen;
7. select STOP to prematurely end the treatment,
8. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
CONTINUOUS delivery METHOD
To start the treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
3. proceed with the emission by continuously pressing the trigger on the
handpiece (or the footswitch): this enables the rapid succession of the
impulses set for the delivery of the treatment;
4. to suspend dispensing, remove pressure on the applicator trigger (or on the
footswitch);
5. to resume the treatment, keep the trigger pressed continuously, as
previously seen;
6. select STOP to prematurely end the treatment,
7. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.
SINGLE delivery method
To start the treatment:
1. place the handpiece on the part to be treated;
2. touch the START button;
3. to proceed with the emission, press the trigger on the handpiece (or the
footswitch);: this enables the emission of a single shot;
4. to deliver new shots, repeatedly press the trigger (or the footswitch);
5. select STOP to terminate the treatment early,
6. or wait for the timer to reset which indicates that the treatment has been
completed and then select the OK button.

SW2050 - SW2051
S
SH
HO
OC
CK
K
M
ME
ED
D
–
–
S
SH
HO
OC
CK
K
M
ME
ED
D
S
SP
P
FT05MI11
18
fig.8
In each delivery mode, during the treatment, shocks performed during the delivery of
the therapy and the time remaining at the end of the therapy are displayed (Fig. 8). It
is calculated on the basis of:
-the number of pulses remaining
-at the pulse frequency
-the way of working.
During the delivery of treatments, it is possible to change the FREQUENCY parameter
except in the SINGLE delivery mode and the INTENSITY parameter.
L
LO
OA
AD
D
In this section it is possible to load a PROGRAM choosing from the customized ones,
following the instructions below:
1. Select LOAD from the FREE PROCEDURES screen in figure 3;
2. If necessary, scroll the list of therapies up or down using the appropriate side
scroll bar;
3. Select the desired customized program in the list of therapies, the button will
appear (figure 9). Press the key to open the treatment;
oOtherwise, press the BACK button to return to the main screen.
At this point it is possible:
omodify the processing data, proceeding as indicated in MODIFY;
osave any modified parameters proceeding as indicated in SAVE;
ostart the treatment, following the START procedure;
opress the BACK button to go back to the main screen;
If you want to delete a custom treatment:
7. press for a few seconds on the name of the selected treatment, a yellow
button will appear with a trash can symbol;
8. Pressing the button depicting the bin leads to the appearance of two other
symbols: a prohibition and an X (figure 9).
9. Press the X to cancel or press the prohibition to proceed with the
elimination.
oPress the BACK button to go back to the main screen.
fig9
This manual suits for next models
2
Table of contents
Other EME Medical Equipment manuals