Empi phoenix User manual

Empi
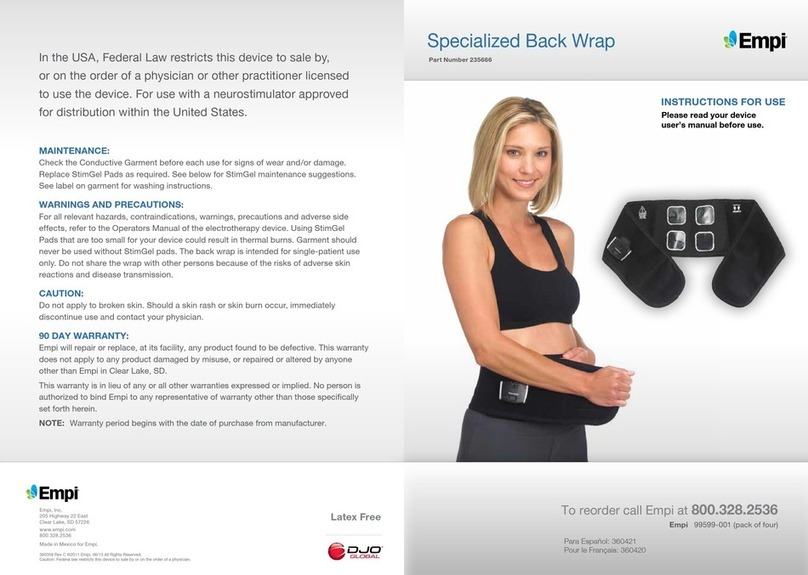
CONDUCTIVE GARMENT
USER’S MANUAL
• Read carefully before using this Garment
• Visit www.djoglobal.com
™

Phoenix Garment User Manual
2
IMPORTANT: Please read the Empi™Phoenix Device
User’s Manual (360413 for USA, 360425 for International)
before use.
Product Overview
The Empi Phoenix Conductive Garment for the thigh is
an accessory to Empi Phoenix device designed to deliver
electrical stimulation to muscles to aid with muscle
recovery and re-education. This specialized garment is a
light-weight, breathable fabric that wraps around the thigh
and is secured with Velcro. The Garment is designed to
provide a convenient application of therapy.
In the USA, Federal Law restricts this device to sale by or
on the order of a physician or other practitioner licensed to
use the device.
Intended Use
The Empi Phoenix Conductive Garment is intended for use with the Empi Phoenix device,
which is indicated for retarding or preventing disuse atrophy, maintaining or increasing
range of motion, and re-educating muscles. The garment provides a conductive pathway
between the Phoenix device and the Phoenix Garment Electrodes.
This Garment is only to be used with the Phoenix device’s NMES P1 and P2 programs
(“Endurance” and “Strength” respectively for the USA, “Disuse Atrophy” and
“Reinforcement” respectively for International).
Table of Contents
Product Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Glossary of Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Warnings and Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Garment Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Environmental Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Storage and Transport Conditions . . . . . . . . . . . . . . . . . . . . . . . . . 6
Electrode Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Ordering . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7

3
Phoenix Garment User Manual
Glossary of Symbols
This device may contain one or more of the following symbols:
Warnings and Precautions
For a full list of relevant contraindications, warnings, precautions and adverse reactions,
refer to the Empi Phoenix Device User’s Manual.
• Single Patient Use Only: Do not share the garment with other persons because of the
risks of adverse skin reactions and disease transmission.
• Not a brace: The specialized garment is not intended to be used as a brace/lower leg
support.
• Use during activity: This garment is intended to be used in a seated position or in
accordance with your clinician’s orders. Muscle contractions caused by the device
could affect walking or balance.
• Application on broken skin: Should a skin rash or skin burn occur, immediately
discontinue use and contact your physician.
• Power connection: Do not connect the garment to an AC power source or other
equipment not specied as safe for the garment. Doing so could result in severe
shock or burns whether or not the garment is attached to the stimulator. The leadwires
should only be connected to the device and the garment.
• Electrodes: The garment should never be used without the three Phoenix Garment
Electrodes. Thermal burns may result from use without all electrodes. Electrodes
should be securely fastened to prevent inadvertent disconnection. Electrodes will
eventually wear out. Check accessories regularly for signs of wear and replace as
needed.
• Skin reactions: On rare occasions, therapy can result in transient skin reactions such
as rash, inammation, irritation or burns. These skin reactions may be the result of
individual sensitivity, the condition of the skin at the onset of treatment, or a reaction to
the materials in the electrodes or garment.
• Safety: The safety and efcacy of the Empi Phoenix system depend on the proper
use and handling of the product and accessories. Use only as prescribed. Keep out of
reach of children. Keep away from open ame.
• Use with other devices/accessories: Use only the Phoenix device that is specially
designed for this garment. Do not use accessories (including electrodes) or devices
manufactured by other companies on this garment. Empi is not responsible for any
consequence resulting from using products manufactured by other companies.
Refer to Instruction Manual/Booklet
Caution: For Prescription Only
Federal law (USA) restricts this device
to sale by or on the order of a physician
(or licensed practitioner).
Precautionary Instructions
Keep Dry
No Latex
Keep Away from Sunlight
Min and Max storage
temperatures.
This Garment is intended for use with Empi Phoenix device indoors in a seated position
at home or in a clinic environment. Follow your health care provider’s directions for more
specic use instructions.
Rx
ONLY
USA +27°
C
+5°C
+80.6
°F
+41.0
°F

Phoenix Garment User Manual
4
Garment Use
1. Lay the garment at with the three conductive
silver buttons facing up as pictured.
2. Remove the blue plastic lining from the grid side
of Phoenix Garment Electrodes and discard.
3. Press the grid side of the Phoenix Garment
Electrodes over the silver buttons within the
appropriate outlines. If you are using the garment
to treat your right leg, place the electrodes in the
grey outlines marked with “Right Leg Electrode
Placement”. If you are using the garment to
treat your left leg, place the electrodes in the
white outlines marked with “Left Leg Electrode
Placement”. The large electrode should only be
placed within the corresponding larger grey/white
dashed outline.
IMPORTANT: Make sure to place ALL THREE
electrodes on the garment before using.
The electrodes must completely cover each
conductive metal button before use.
STOP! If you are using the garment for your
LEFT leg, you should have it set up to look like
Fig. 1
If you are using the garment for your RIGHT leg,
you should have it set up to look like Fig 2.
4. Remove the remaining clear liner from the face
of the electrodes and SAVE for re-application to
Phoenix Garment Electrodes in between uses.
5. Sit on the edge of a rm, stable chair with your
knee slightly bent and your foot at on the oor.
If your clinician instructed you to use the device
and garment in a different setting or position,
follow your clinician’s instructions.
Fig. 1 Left
Fig. 2
Right

5
Phoenix Garment User Manual
6. Place the wrap over your thigh with the Empi logo facing you and centered on your
thigh. The far edge of the garment should be just above your kneecap. The underside
of the garment also indicates the proper orientation of the garment in relation to your
knee.
IMPORTANT: Do not use this garment on any body part other than the quadriceps/
thigh.
7. Wrap the two straps underneath and around your
thigh and afx the Velcro™ on the softer fabric
“landing strip”.
With the Phoenix Garment Electrodes aligned
over your thigh/quadriceps muscles, gently
apply pressure on the top section of the wrap to
secure the electrodes against the skin. Do not
slide or pull the garment to readjust its position
while wearing it as the electrodes may roll up
underneath the garment. To adjust, remove the
garment completely and reposition.
8. Connect the black plastic leadwire connector to
the garment by sliding it onto the port as shown.
You should hear an audible click.
9. Attach the Empi Phoenix device by inserting
both black leadwire heads into the ports at the
top of the device. Press rmly to ensure that the
leadwire is fully inserted into the device.
10.Begin therapy as directed by your clinician.
Unless otherwise directed by your healthcare
provider, you should be seated during therapy.
You can holster the device in the garment by
attaching the device’s belt clip and sliding it into
the at, wide strap in the middle section of the
garment on top of your thigh. Do not stand up
while the device is in place on the garment.
When your session is complete, turn the device
off.
IMPORTANT: This Garment is only to be used
with the Phoenix device programs P1 and P2.
Phoenix device programs P3 and P4 should be
used with the direct leadwires and electrodes
provided with the device.

Phoenix Garment User Manual
6
Storage
• Gently remove the garment. The Phoenix Garment Electrodes will lift off of your skin
and stay attached to the wrap. Electrodes should remain on wrap for storage. If the
electrodes remain on your skin instead of the garment, remove them from your skin
and place them back on the garment in the appropriate location.
• Replace the saved liner on the electrodes. Be sure to place the “ON” side against the
Phoenix Garment Electrode surface.
• Store the garment in its original bag until next treatment. Re-applying the liners, folding
up after each use, and storing the wrap in re-sealable bag will prolong the life of the
Phoenix Garment Electrodes.
Maintenance
Check the specialized garment before each use for signs of wear and/or damage. Make
sure that the electrodes have good tack so that they will stick well to the skin.
Replace the Phoenix Garment Electrodes as required. See “Electrode Use” section for
Phoenix Garment Electrodes maintenance suggestions. When electrodes are no longer
useful, dispose of them in a receptacle out of the reach of children or pets.
If needed, you may wash the Phoenix Garment. See label on wrap for washing
instructions. Make sure the garment is dry before using it. With proper care, this garment
is designed to have a life of six months.
Environmental Conditions
Operating Conditions
• Temperature Range: 10º C to 40º C (50º F to 104º F)
•Atmospheric Pressure Range: 50kPA to 106 kPa
• Relative Humidity Range: 30% to 75%
Storage and Transport Conditions
•Temperature Range: -40º C to 70º C (-40º F to 158º F)
• Atmospheric Pressure Range: 50kPA to 106 kPa
• Relative Humidity Range: 10% to 90%
Electrode Use
You can extend the use of your Phoenix Garment Electrodes by following these
suggestions:
• Cleanse, rinse and dry skin prior to use.
• Store electrodes as indicated above and on electrode packaging.
• If electrode gel becomes dry, adding a few drops of water to each side of the electrode
may help restore adhesive gel of electrode. Let the electrode rest to regain tack before
applying to skin.

7
Phoenix Garment User Manual
90 Day Warranty
Empi will repair or replace, at its facility, any product found to be defective within 90 days
of purchase. This warranty does not apply to any product damaged by misuse, or repaired
or altered by anyone other than Empi in Clear Lake, SD, or an ofcially approved DJO
Global service center.
This warranty is in lieu of any or all other warranties expressed or implied. No person is
authorized to bind Empi to any representative of warranty other than those specically set
forth herein.
NOTE: Warranty period begins with the date of purchase from manufacturer.
Ordering
To reorder call: Empi at 800.328.2536 (USA Only)
Empi Phoenix Garment – P/N 235684
Phoenix Garment Electrode Pack – P/N 199695
(pack of two 4”x2.75” (10 x 7 cm) and one 6”x3.5” (15 x 9 cm))
Manufactured in Mexico for:
Empi, Inc.
205 Hwy 22 East
Clear Lake, SD 57226 USA
+1 651 415 9000
360412, Rev. C © 2014 Empi
EU Authorised Representative
MDSS GmbH
Schiffgraben 41
30175 Hannover
Germany

Empi
COMPLETE
ELECTROTHERAPY
SYSTEM
USER’S MANUAL
• Read this manual carefully before operating the Phoenix™
• Visit us at www.djoglobal.com
™

Phoenix User’s Manual
2
Table of Contents
1. Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2. Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
2.1 Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
2.2 Use Environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
3. Explanation of Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
4. Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
4.1 Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
4.2 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
4.3 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.4 Dangers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
4.5 Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
5. How Does Electrotherapy Work? . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6. Usage Guidelines. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
6.1 Program Descriptions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.1.1 Endurance – P1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
6.1.2 Strength – P2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
6.1.3 Modulated TENS – P3. . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
6.1.4 Edema – P4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
6.2 Choice of the Appropriate Program . . . . . . . . . . . . . . . . . . . . . . . . . 14
6.3 Planning of Stimulation Sessions . . . . . . . . . . . . . . . . . . . . . . . . . . 15
6.4 Electrode Positions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
6.4.1 Use of the Empi Phoenix Thigh Garment . . . . . . . . . . . . . . . . . . . 15
6.5 Stimulation Positions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
6.6 Adjusting Stimulation Energies . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
7. Operating Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
7.1 Description of the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
7.2 Kit Composition and Accessories Description . . . . . . . . . . . . . . . . . . . .18
7.3 Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
7.3.1 Insertion/replacement of the batteries . . . . . . . . . . . . . . . . . . . . . 19
7.3.2 Connection of the lead wires to the device . . . . . . . . . . . . . . . . . . 19
7.3.3 Placement and care of the electrodes . . . . . . . . . . . . . . . . . . . . . 20
7.3.4 Connection of the lead wires to the electrodes . . . . . . . . . . . . . . . . 20
7.3.5 Use of the Empi Phoenix Thigh Garment (optional). . . . . . . . . . . . . . .20
7.4 Operation of the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
7.4.1 LCD Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
7.4.2 Operation Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
7.4.3 Operating Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
7.4.4 Pause Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
7.4.5 End of Treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
7.4.6 Use of the Hand Switch (optional) . . . . . . . . . . . . . . . . . . . . . . . 22

3
Phoenix User’s Manual
7.5 Meaning of Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
7.5.1 Intensity Lock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
7.5.2 Sequence Indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
7.5.3 Work/Rest Indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
7.5.4 Timer Indication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
7.5.5 Low Battery Indicator . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
7.5.6 Open Circuit Icon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
8. Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
9. Device Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
9.1 Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
9.2 Cleaning and Calibration. . . . . . . . . . . . . . . . . . . . . . . . . . . .26
9.3 Repair . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
9.4 Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
9.5 Transportation and Storage Conditions . . . . . . . . . . . . . . . . . . . . 27
9.6 Expected Life and Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . 27
10. Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
11. Clinician Only Section . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
11.1 Compliance Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
11.2 Program Lock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
11.3 Ordering Information for Clinicians . . . . . . . . . . . . . . . . . . . . . . .29
12. Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
13. Technical Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
14. Guidance and Manufacturer’s Declaration – Electromagnetic Emissions. . . . . .34
15. Additional Information on Electrode Placement for Knee Treatment . . . . . . 38
16. Quick Start Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Phoenix User’s Manual
4
1. Foreword
The Empi Phoenix is a multifunctional electrotherapy device that provides two channels of
neuromuscular electrical stimulation (NMES), transcutaneous electrical stimulation (TENS),
or pulsed DC (Edema treatment). This wide-ranging capability allows the patient to receive
electrotherapy throughout the recovery cycle using a single device. Its simplified programming
makes the device convenient for home use: after placing the electrodes and selecting the
program as prescribed by a healthcare professional, the patient only needs to increase the
intensity to begin therapy.
The Phoenix device’s NMES Endurance and Strength programs utilize an electrical stimulus
that, when properly applied, activates specific muscles or muscle groups to help treat disuse
atrophy and re-educate muscles. The pre-set programs are designed to provide therapeutic
benefit while minimizing complexity for the patient and clinician. The device can be paired with
the Empi Phoenix Conductive Garment for the thigh, which is designed to make treatment of
the knee/quadriceps with NMES easier for the patient and clinician.
The Phoenix device also includes a traditional TENS program for pain management and a
pulsed DC Edema program to increase local blood circulation and reduce edema (swelling).
Read this User Manual carefully before using the Phoenix device. Pay particular
attention to the Safety Information in Section 4 and additional warnings throughout
the manual.
Caution: Federal law restricts this device to sale by or on the order of a practitioner
licensed by the law of the State in which he/she practices to use or order the use of
the device.

5
Phoenix User’s Manual
2. Intended Use
2.1 Indications for Use
As an NMES device, indications are for the following conditions:
• Retardingorpreventingdisuseatrophy
• Maintainingorincreasingrangeofmotion
• Re-educatingmuscles
• Relaxationofmusclespasms
• Increasinglocalbloodcirculation
As a TENS device, indications are for the following conditions:
• Symptomaticreliefandmanagementofchronic,intractablepain
• Adjunctivetreatmentforpost-surgicalandpost-traumaacutepain
• Reliefofpainassociatedwitharthritis
As a pulsed current device, indications are for the following conditions:
• Reductionofedema(undernegativeelectrode)
• Reductionofmusclespasm
• Inuencinglocalbloodcirculation(undernegativeelectrode)
• Retardationorpreventionofdisuseatrophy
• Facilitationofvoluntarymotorfunction
• Maintenanceofincreaseofrangeofmotion
2.2 Use Environment
The Empi Phoenix device is a prescription device in the USA and is intended to be used
following the directions of a healthcare provider. The device should be used indoors and may
be used in a healthcare facility setting or by a patient or lay operator in a home environment.

Phoenix User’s Manual
6
3. Explanation of Symbols
The following symbols are used either in this user manual, on the device packaging, or on the
device label. They may also appear on an accessory.
Symbol Explanation
Reference number; part number
Lot number
Follow instruction for use
Type BF applied parts
Keep the device dry
Keep the device away from sunlight
Protected against solid foreign objects of 12.5 mm (0.5 in) diameter and greater
Protected against vertically falling water drops when enclosure tilted up to 15°
Minimum and maximum temperature indications to respect
Prescription only (USA)
C
E
T
L
C
L
A
S
S
I
F
I
E
D
9900900
ETL Classified C US, 9900900, Electronic Testing Lab, indicates product meets
US and Canadian product safety standards. This device Conforms to AAMI Std.
ES60601-1. Certified to CAN/CSA Std. C22.2#60601-1.
Manufacturing year
Manufacturer name and address
Power/Pause
Dangerous voltage
Lead wires comply with the Performance Standard for electrode lead wires
(21 CFR part 898)
7
Phoenix User’s Manual
4. Safety Information
This section includes Contraindications, Warnings, Precautions, Dangers, and Adverse
Reactions.
Implanted electronic devices. Do not use the Empi Phoenix device on patients who have a
cardiac pacemaker, implanted defibrillator, or other implanted electronic device, because this
may cause electric shock, burns, electrical interference, or death.
TENS for undiagnosed pain. Do not use the Empi Phoenix device as a TENS device (P3) on
patients whose pain syndromes are undiagnosed.
Consult with physician. Consult with the patient’s physician before using the Empi Phoenix
device, because the device may cause lethal rhythm disturbances to the heart in susceptible
individuals.
Skin condition. Apply stimulation only to normal, intact, clean, healthy skin.
Long term effects. The long-term effects of chronic electrical stimulation are unknown.
Stimulation location
Stimulation over neck or mouth. Do not apply stimulation over the patient’s neck
(especially the carotid sinus) or the patient’s mouth, because this could cause severe muscle
spasms resulting in closure of the airway, difficulty in breathing, or adverse effects on heart
rhythm or blood pressure.
Stimulation across chest. Do not apply stimulation across the patient’s chest, because the
introduction of electrical current into the chest may cause rhythm disturbances to the patient’s
heart, which could be lethal.
Across the head. Since the effects of stimulation of the brain are unknown, stimulation
should not be applied across the head, and electrodes should not be placed on opposite
sides of the head.
Stimulation over compromised skin. Do not apply stimulation over open wounds or
rashes,oroverswollen,red,infected,orinamedareasorskineruptions(e.g.,phlebitis,
thrombophlebitis, varicose veins).
Stimulation near cancerous lesions. Do not apply stimulation over, or in proximity to,
cancerous lesions.
Stimulation over metallic implants. Do not apply stimulation directly over implanted
metallic devices, because this may cause shock or burns.
Stimulation over eyes. Do not apply stimulation directly on the eyes.
Environment
Electronic monitoring equipment. Do not apply stimulation in the presence of electronic
monitoring equipment (e.g., cardiac monitors, ECG alarms), which may not operate properly
when the electrical stimulation device is in use.
Bath or shower. Do not apply stimulation when the patient is in the bath or shower. Do not
apply stimulation in humid atmosphere exceeding 75% of relative humidity.
4.1 Contraindications
4.2 Warnings

Phoenix User’s Manual
8
Sleeping. Do not apply stimulation while the patient is sleeping.
Driving or operating machinery. Do not apply stimulation while the patient is driving,
operating machinery, or during any activity in which electrical stimulation or involuntary muscle
contraction can put the patient at risk of injury.
Electrosurgical equipment or defibrillators. Disconnect the Empi Phoenix stimulation
electrodes before using electrosurgical equipment or defibrillators. Otherwise skin burns may
be caused below the electrodes and the Empi Phoenix device might be destroyed.
Magnetic Resonance Imaging. Do not wear electrode or the Empi Phoenix device during
Magnetic Resonance Imaging (MRI) scans as this may result in metal overheating and causing
skin burns in the area of the electrode.
Flammable or explosive environment. Do not use the Empi Phoenix device in areas
where there is a risk of fire or explosion, such as oxygen-rich environments, in the vicinity of
ammableanaesthetics,etc.
Power supply. Never connect stimulation cables to an external power supply as there is a
risk of electric shock.
Near other equipment. Do not use the Empi Phoenix device beside or stacked on top of
any other equipment. If you must use it side by side or on top of another system, you should
check that the Empi Phoenix device works properly in the chosen configuration.
Miscellaneous
Garment and electrodes for single patient. Do not share electrodes or garments with
other persons. All users should have individual set of electrodes to prevent undesirable skin
reactions or disease transmission.
Accessories. Use this device only with the leads, electrodes, and accessories recommended
by Empi. Use of other accessories may adversely affect the performance of the device or may
result in stronger electromagnetic emissions or reduce the electromagnetic immunity of the
Empi Phoenix device.
No Modification. No modification of the equipment is allowed.
4.3 Precautions
Supervision. Use this device only under the continued supervision of a licensed practitioner.
Electrode placement and stimulation settings should be based on the guidance of the
prescribing practitioner.
Pregnancy. The safety of electrical stimulation during pregnancy has not been established.
Skin irritation. Some patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrical conductive medium (gel). The irritation may be reduced by
using an alternate conductive medium or alternate electrode placement. Some patients may
experience redness under the electrodes after a session. This redness usually disappears
within a few hours. Advise the patient to consult the clinician if the skin redness does not
disappear after a few hours. Do not start another stimulation session in the same area if the
redness is still visible. Don’t scratch the redness area.
Heart disease. Patients with suspected or diagnosed heart disease should follow
precautions recommended by their physicians.
Epilepsy. Patients with suspected or diagnosed epilepsy should follow precautions
recommended by their physicians.
Internal bleeding. Use caution when the patient has a tendency to bleed internally, such as
following an injury or fracture.
After surgery. Use caution following recent surgical procedures when stimulation may disrupt
the patient’s healing process.
Over uterus. Use caution if stimulation is applied over the menstruating or pregnant uterus.

9
Phoenix User’s Manual
Lack of sensation. Use caution if stimulation is applied over areas of skin that lack normal
sensation. Don’t apply stimulation on patient unable to express themselves.
Hot casing or batteries. Under extreme use conditions, some parts of the casing might
reach up to 109 °F (43 °C). Use caution when manipulating the batteries right after device use
or when holding the device. There is no particular health risk associated with this temperature
besides your comfort.
Children. Keep this device out of the reach of children.
Electrode size. Do not use electrodes with an active area less than 16 cm2, as there will be a
risk of suffering a burn injury. Caution should always be exercised with current densities more
than 2mA/cm2.
Strangulation. Do not wrap leadwires around your neck, and keep them out of the reach of
children. Strangulation may result from entanglement in the leadwires.
Tripping. Care should be used to avoid tripping on lead wires.
Damaged device or accessories. Never use the Empi Phoenix device or any of its
accessories if it is damaged (case, cables, etc.) or if the battery compartment is open as there
is a risk of electric shock. Carefully inspect the lead wires and connectors prior to each use.
Inspect electrodes. Inspect electrodes before each use. Replace electrodes when they
begin to deteriorate or lose adhesion. Poor contact between the electrodes and the patient’s
skin increases the risk of skin irritation or burns. Electrodes will last longer if used and stored
according to instructions on electrode packaging. Attach the electrodes in such a way that
their entire surface is in contact with the skin.
Foreign bodies. Do not allow any foreign bodies (soil, water, metal, etc.) to penetrate the
Empi Phoenix device and the battery compartment.
Garment. Do not use the Empi Phoenix Thigh Garment in proximity of fire or excessive heat
sources due to the risk of fire. Make sure that the electrodes cover the metal connectors on
the Phoenix garment before use to avoid shocking, skin irritation, and burns.
Batteries. Do not carry batteries in a pocket, purse, or any other place where the terminals
could become short-circuited (e.g. by way of paper clip). Intense heat could be generated and
injury may result.
Heat and cold products. The use of heat or cold producing devices (e.g. electric heating
blankets, heating pads or ice packs) may impair performance of the electrode or alter the
patient’s circulation/sensitivity and increase the risk of injury to the patient.
Pulled muscles. Do not apply electrodes over pulled muscles. Using the stimulator on
a previously extended muscle might further pull such muscle. The higher the stimulation
intensity, the higher the risk to further overextend such muscle.
DC Component. The Empi Phoenix waveforms may contain a DC component (only for
Edema program P4). Always use Empi electrodes with a minimum active area of 16 cm2
(including Empi square (2” x 2”) StimCare electrodes). Use of an electrode with an area less
than 16 cm2can cause burns when the unit is used at higher intensities. Consult your clinician
prior to using any electrode less than 16 cm2.
This DC component for the Edema program (P4) is equivalent to 266 µA DC for all intensities
above 4 mA.
Additional Precautions for TENS (P3)
• TENSisnoteffectiveforpainofcentralorigin,includingheadache.
• TENSisnotasubstituteforpainmedicationsandotherpainmanagementtherapies.
• TENSdeviceshavenocurativevalue.
• TENSisasymptomatictreatmentand,assuch,suppressesthesensationofpainthat
would otherwise serve as a protective mechanism.
• EffectivenessofTENSishighlydependentuponpatientselectionbyapractitioner
qualified in the management of pain patients.

Phoenix User’s Manual
10
4.4 Dangers
Electrodes. Any Empi Electrode with a minimum active area of 16 cm2may be used
with this device. This includes Empi 2” round and 2” square StimCare electrodes. Use
of an electrode with an area less than 16 cm2can cause burns when the unit is used at
higher intensities. Consult your clinician prior to using any electrode less than 16 cm2.
Dangerous voltage. Stimulus delivered by the waveforms of the Phoenix device,
in certain configurations, will deliver a charge of up to 20 microcoulombs (µC) or
greater per pulse and may be sufficient to cause electrocution. Electrical current of
thismagnitudemustnotowthroughthethoraxbecauseitmaycauseacardiac
arrhythmia.
4.5 Adverse Reactions
• Patientsmayexperienceskin irritation and burns beneath the stimulation electrodes
applied to the skin.
• Patientsmayexperienceheadache and other painful sensations during or following
the application of electrical stimulation near the eyes and to the head and face.
• Patientsshouldstop using the device and should consult with their physicians if they
experience adverse reactions from the device.
11
Phoenix User’s Manual
5. How Does Electrotherapy Work?
The principle of electrotherapy is to stimulate nerve fibers by means of electrical impulses
transmitted by electrodes. The NMES electrical pulses generated by the Empi Phoenix
stimulators are high-quality pulses that have been clinically tested and offer safety, comfort,
and efficiency. These electrical pulses can:
• Stimulatemotorpointsoftargetmuscles,causingamusclecontraction.Thiscanhelp
re-educate and strengthen your muscles following an injury or surgery. This is called
neuromuscular electrical stimulation (NMES). The Empi Phoenix programs P1 and P2
are NMES programs.
• Managepain.Theelectricalpulsesblockthepainsignalsentfromtheaffectedarea
on your nerve pathways. This is called the “Gate Theory” of pain control, and this form
of electrotherapy is called transcutaneous electrical nerve stimulations (TENS). The
Empi Phoenix program P3 is a TENS program.
• Increaselocalbloodcirculation,helpingtoreduceswellingoredema.Theelectrical
currentcanaffectthemovementofuidthroughtissue,andincreasingbloodowcan
help increase healing. This therapy can be achieved using a pulsed direct current. The
Empi Phoenix program P4 is a pulsed, direct-current program.
During voluntary activity, the brain sends a command to the nerve fibers in the form of an
electrical signal to give the order to move. This signal is then transmitted to the muscular
fibers, which contract. The principle of electrotherapy emulates the process observed during
a voluntary contraction. In other words, the muscle cannot distinguish whether the command
comes from the brain or from the stimulator. The parameters of the Empi Phoenix programs
(number of pulses per second, contraction time, rest time, total program time) subject the
muscles to different types of work.
In fact, different types of muscular fibers may be distinguished according to their respective
contraction speed: slow, intermediate, and fast fibers. Fast fibers would predominate in a
sprinter, while a marathon runner would likely have more slow fibers. With a good knowledge
of human physiology and well‐designed stimulation programs, muscular work can be directed
very precisely towards the desired goal (muscular re-education, relaxation of muscle spasm,
painmanagement,increasedbloodow,maintaining,orincreasingrange‐of‐motion,etc.)
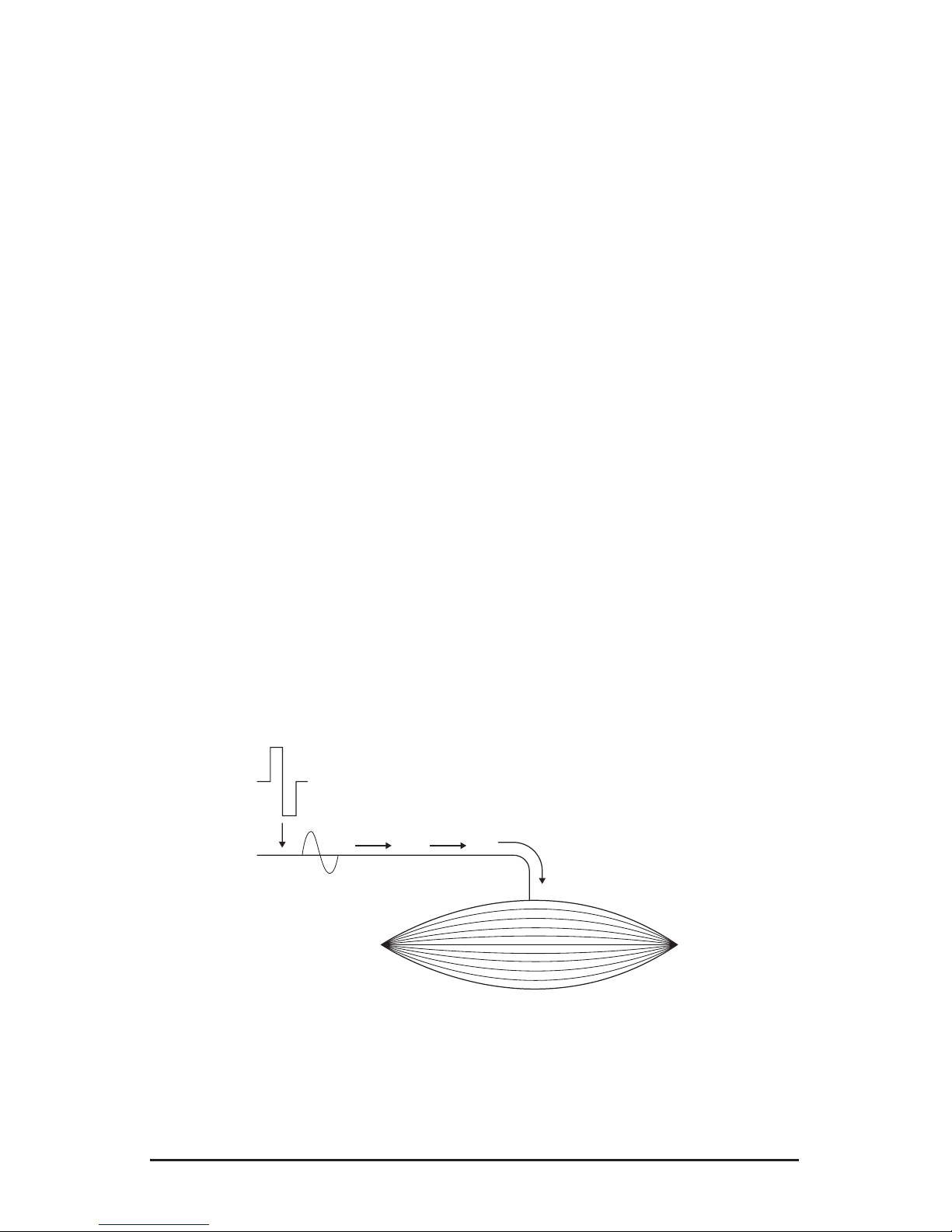
Transmission
of the excitation
Electrical pulse
Excitation
Motor
nerve
Elementary Mechanical Response - Twitch
Stimulated
muscle

Phoenix User’s Manual
12
6. Usage Guidelines
6.1 Program Descriptions
The choice of a program is determined by the injured body parts or joints. The appropriate
stimulation programs (e.g., Endurance, Strength, TENS, or Edema) and frequency of the
program(s) are determined by the medical professional. Consult your medical professional to
be sure to understand the Empi Phoenix device.
6.1.1 Endurance – P1
The Empi Phoenix Endurance program focuses on generating a medium muscle contraction.
This working level is maintained over a long time period (20 minutes per session). The
Endurance program specifically activates the aerobic metabolism of the fibers during
the stimulation session. The purpose is to increase the time for the muscle to maintain a
medium contraction or the average power level for extended periods of time. This program
is recommended for use before and after the surgery as prescribed by your medical
professionals.
P1 begins with a two-minute Warm-Up phase, which will count down on the screen. The
Warm-Up phase will cause your muscles to twitch but not contract. Set the device intensity
to a comfortable level during Warm-Up. Once the Work phase begins, the intensity will
automatically decrease by half. You can then adjust the intensity to provide a comfortable
but strong muscle contraction. The program finishes with a Cool-Down phase similar to the
Warm-Up phase.
ENDURANCE (P1)
Waveform Symmetrical square biphasic asynchronous
WARM UP SETTING
Treatment time 2 min
Cycling type Continuous
Pulse duration 300µs
Frequency - warm up 6 Hz
WORK PHASE SETTING
Treatment time 15 min
Cycling type Intermittent
Pulse duration 300µs
Frequency - work 35 Hz
Frequency - rest 4 Hz
Work time 6 sec
Rest time 7 sec
Ramp up time work 1.5 sec
Ramp down time work 0.75 sec
Ramp up time rest 0.5 sec
Ramp down time rest 0.5 sec
Table of contents
Other Empi Fitness Equipment manuals
Popular Fitness Equipment manuals by other brands

DAY 1 Fitness
DAY 1 Fitness BODY BAR MANUAL & EXERCISE GUIDE

Nordic
Nordic Vibroplate manual
SPORTSTECH
SPORTSTECH BRT100 user manual

Sunny Health & Fitness
Sunny Health & Fitness SF-XF922059 user manual

BH FITNESS
BH FITNESS GlobalGym plus G152X Instructions for assembly and use
Movement
Movement BOLT PLATE LOADED manual