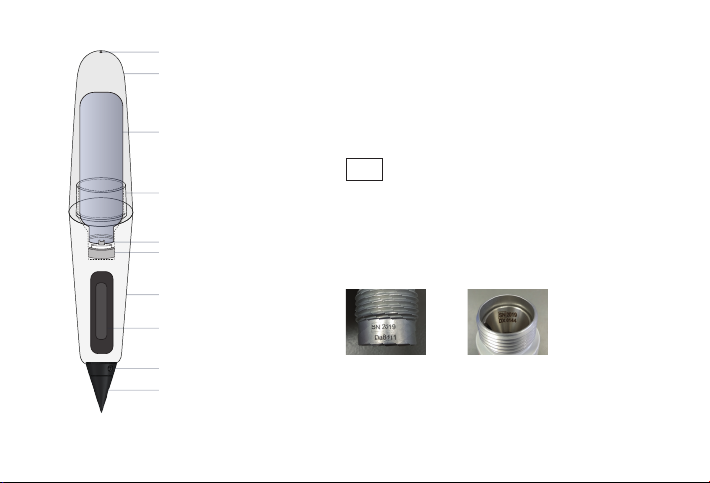
8
Contraindications:
• Unstable diabetes
• Skin conditions, e.g. skin tumors, esanthema, open
wounds, solar hyperkeratosis
• Unexplained, suspicious liver spots and moles
• Cancer tissues and malignant tumors
The contraindications related to temporary
conditions:
• Infections accompanied by fever
• Acute chemotherapy or radiotherapy from four weeks
before beginning the therapy to four weeks after
finishing the therapy
• Pregnancy or breastfeeding
• Cold intolerance
• Vascular insufficiency
Reduce possible side-effects:
Although cryotherapy is a relatively low-risk procedure, some
side effects may occur as a result of the treatment.
They include:
• Permanent changes in pigmentation. Both
hypopigmentation (lightening of the skin) and
hyperpigmentation (darkening of the skin) may occur after
cryotherapy. Both generally last a few months, but can be
longer lasting. Avoid freezing the basal cell layer where
melanocytes (pigment producing cells) are located.
• Sensory impairment. Though rare, damage to nerves is
possible, particularly in areas where they lie closer to the
surface of the skin, such as the fingers, the wrist, and the
area behind the ear. Reports suggest this will disappear
within several months.
• Spattering of the cryogen during spraying, when the
end nozzle freezes. The innovation of CryoPen is the
direct application of nitrous oxide under high pressure
(49-53 bar for a cartridge temperature of 66-72°F/19-
22°C). This high pressure jet may cause minor shards
of frozen humidity in the air blown away in a circle of
approximately 30cm of diameter. They will thaw the
moment they would eventually touch healthy skin.
• Hair loss. Hair follicles are easily damaged by cryosurgery
and permanent hair loss is not uncommon.