hatch A50B User manual

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
HATCH GROUP INC.
Pressure Steam Sterilizer
Autoclave A50B 3L Operation Manual V1.0

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
2
Catalogue
Catalogue.............................................................................................................................................................................2
Scope of Use........................................................................................................................................................................3
User Guide...........................................................................................................................................................................3
1. Brief introduction............................................................................................................................................................7
2. Specifications..................................................................................................................................................................9
3. Installation.....................................................................................................................................................................11
4. Adjusting.......................................................................................................................................................................15
5. Introduction of preset programs ....................................................................................................................................15
6. User interface description..............................................................................................................................................17
7. Instructions of operation................................................................................................................................................23
8. Errors list.......................................................................................................................................................................28
9. Maintenance..................................................................................................................................................................31
10. Quality assurance ........................................................................................................................................................37
11. Accessories..................................................................................................................................................................37
12. appendix......................................................................................................................................................................38
Appendix 1 : Inspection items and results.........................................................................................................................38
Appendix 2: Print record sample and instructions.............................................................................................................38
Appendix 3:Explanation of each stage...........................................................................................................................39

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
3
Scope of Use
This manual is applicable to the tabletop pressure steam sterilizers.
Model:A50B
Please read this manual carefully before use to ensure that the equipment is safe and reliable for your
service!
Please keep this manual during the life of the equipment. If the manufacturer has any necessary
updates, ensure that all updates received are kept as attachments with this manual.
When the place of use or the unit of use of the equipment changes, the manual must be transferred or
handed over as part of the whole equipment.
Please read this manual carefully before using the equipment,be familiar with the
operati
on and safety instructions of the equipment. Do not use instruction that is not from the
manufacturer for reference.
We reserve the right to change the design without prior notice.The information contained in this
manual is current at the time of publication.
User Guide
For safe and reliable use of the equipment, please be sure to pay attention to these instructions.
Safety Caution:
The machine is equipped with some necessary safety measures, in order to avoid causing harm, it is
strictly prohibited to terminate or destroy these safety measures.
Important Notice
Please read this instruction carefully before use.
The equipment must be operated and maintained by professional personnel who must have received
good training.

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
4
For normal operation of the equipment, please keep the equipment clean, do not rinse or water the
equipment;
The maintenance of the equipment must be carried out by the personnel authorized by the
manufacturer, who have passed the corresponding training of the manufacturer;
Equipment accessories or attachments can only be obtained from the manufacturer, otherwise the safe
and effective operation of the equipment cannot be guaranteed.
Emergency handling:
If an emergency is found, it shall be handled as follows:
⚫Turn off the power switch; ⚫
Unplug.
Product responsibilities:
Without the written permission of the manufacturer, it shall not be modified or used beyond the scope.
The manufacturer shall not be liable for any damage caused thereby.
Symbol description:
Special attention should be paid to the danger, warning and attention in this manual
:
Indicates potential hazard to personnel and equipment and must be strictly observed.
:
Indicates potential hazard to personnel and must be strictly observed.
:
The potential harm to the equipment must be taken seriously.
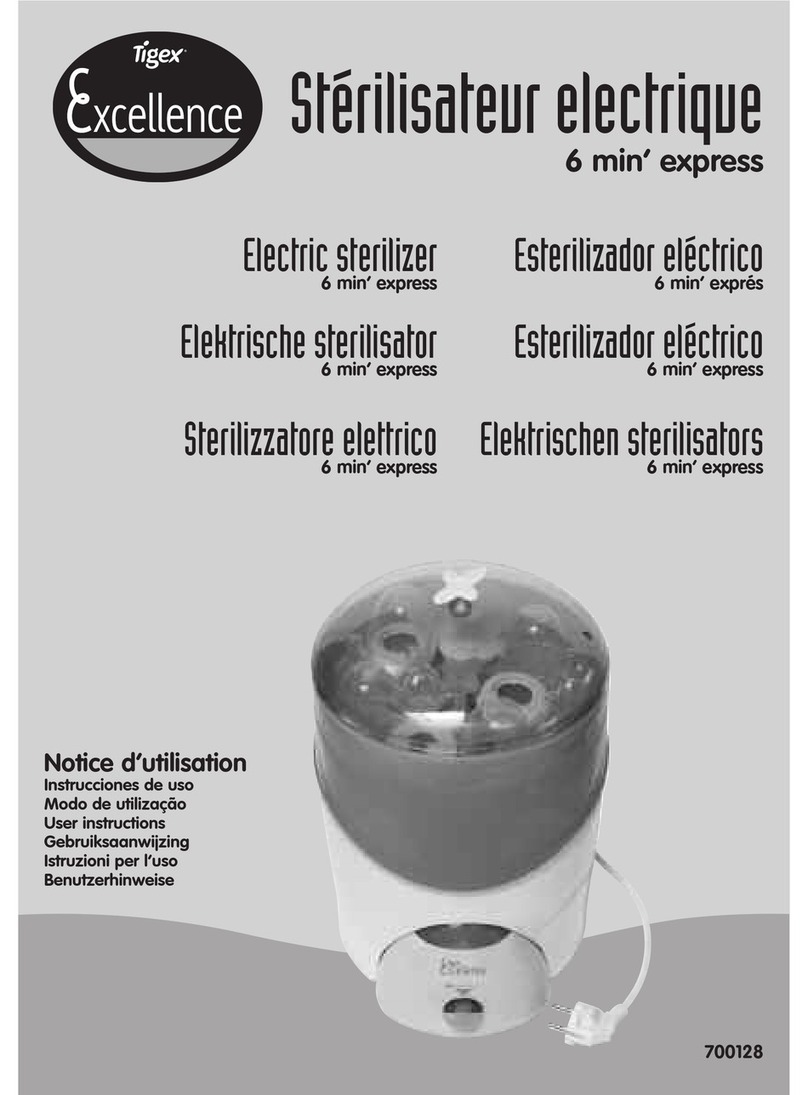
Protective grounding of TypeⅠequipment
Watch for burns on hot surfaces
Refer to manual
Face up
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
5
No rolling
Stack cannot exceed 2 layers
Temperature range 5℃~40℃
Relative humidity is no greater than 80%
keep dry
Safety Notices:
This sterilizer is only suitable to sterilize moisture- and heat-resistant medical devices, not okay
for oils and powders, such as petroleum jelly and agar.
This sterilizer cannot be used for sterilization of liquids or liquids sealed with closed containers
(especially glassware), which may cause the containers to burst, endangering the safety of
persons and equipment.
Chloride ion is an important factor causing corrosion of stainless steel. If you use this sterilizer
to sterilize items which contains chloride ions, you must rinse the sterilization chamber with
clean water after each cycle to prevent the deposited chloride ions from corroding the pot and
extending the service life of the equipment.
When you see the symbol anywhere on the device, it means that the surface temperature
is high, avoid touching to avoid burns.
This equipment complies with the emission and immunity requirements of Class A equipment
specified in GB / T 18268. It may cause interference when used in home or similar environment,
and protective measures are required.
It is recommended to evaluate the electromagnetic environment before using the equipment. It
is forbidden to use this device near strong radiation sources (such as unshielded radio frequency
sources), otherwise it may interfere with the normal operation of the device.

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
6
When an unexpected situation occurs during the use of the product, if the device alarms or other
abnormal conditions occur,please immediately cut off the power supply ofthe device, and check
and correct according to the "abnormal conditions" chapter of the manual.
Necessary monitoring shall be carried out in accordance with relevant national and regional
regulations. After putting the monitoring substance (such as chemical indicator or biological
indicator) into the equipment, run the corresponding program to monitor the sterilization effect,
and judge according to the result. If it fails, find the cause or contact the manufacturer.
A three-hole socket (220 ±22VAC / 16A / 50Hz) must be used to ensure that the socket is reliably
grounded. Do not place the device where it is difficult to disconnect the power.
Do not use other power supplies with different voltages or frequencies.
Do not touch the plug and socket with wet hands.
Do not damage, modify, pull, excessively bend or twist the power cord, and do not place heavy
objects on the power cord.
Please do not place the sterilizer on an unstable workbench, such as a shaking table, a sloping
surface or a place where it will vibrate.
Do not block or cover the sterilizer door, vents or heat dissipation windows.
Please do not place anything on the sterilizer.
If the sterilizer is not used for a long time due to various reasons, the power cord plug should be
disconnected from the power supply and placed in a dry and cool place.
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
7
1. Brief introduction
LF series high-pressure steam sterilizer is specially designed and manufactured by for clinics, hospital
departments, laboratories and other occasions requiring frequent sterilization.Used by a physician or
professional.The sterilizer uses microprocessor intelligent control, man-machine interface, easy to
operate, safe and reliable. The parameters and states in the process of operation are displayed
dynamically by the LCD screen. Automatic fault diagnosis, over temperature, over pressure automatic
protection, ensure the effectiveness of sterilization and disinfection.
1.1 Product categories:
The product is classified as class I type B equipment according to electrical safety;
The product is classified as type B equipment according to YY/T 0646 "small steam sterilizer -
automatic control" standard.
This product is classified as class A equipment according to the electromagnetic compatibility GB/T
18268 standard.
1.2 Product structure:
This product mainly consists of sterilization chamber, sterilization chamber door and seal ring, steam
generator, condenser and fan, vacuum pump, water pump, solenoid valve, sensor, heating ring,
bacteria filter, piping system, power supply, control system, accessories (tray, tray rack, tray handle).
1.3 Range of application
It is used for sterilizing medical instruments which are resistant to heat and humidity.
Don't sterilize the liquid! And ensure that the sterilized equipment can withstand heat and
humidity.
1.4 Sterilization principle, main sterilization factors and strength
1.4.1 Sterilization principle
This equipment is suitable for the vacuum pump to exclude the cold air in the sterilizing room,
take saturated steam as the sterilizing factor, and use the characteristics of high latent heat and high
penetrability of saturated steam to sterilize the equipment.
1.4.2 Sterilization factor and strength
1)sterilization factor and strength of the sterilization procedure at 134℃: the temperature of
saturated water vapor is between 134℃and 137℃, and the temperature difference between each

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
8
point in the sterilization chamber at the same time is no more than 2℃, and the maintenance time is
4min;
2)sterilization factor and strength of the sterilization process at 121℃: the temperature of
saturated water vapor is between 121℃and 124℃, and the temperature difference between each
point in the sterilization chamber at the same time is no more than 2℃, and the maintenance time is
20min.
1.5 Visual section of the device
1
Touch Screen
7
Socket
13
Switch sensor
2
Handle
8
Ventage
14
Door locker
3
Printer
9
Outlet of waste water tank
15
Outlet of waste water tank
4
Clean water tank
10
Outlet of clean water tank
16
Door seal
5
Power switch
11
Safety valve
17
Button battery housing
6
Fuse
12
SD card port
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
9
2. Specifications
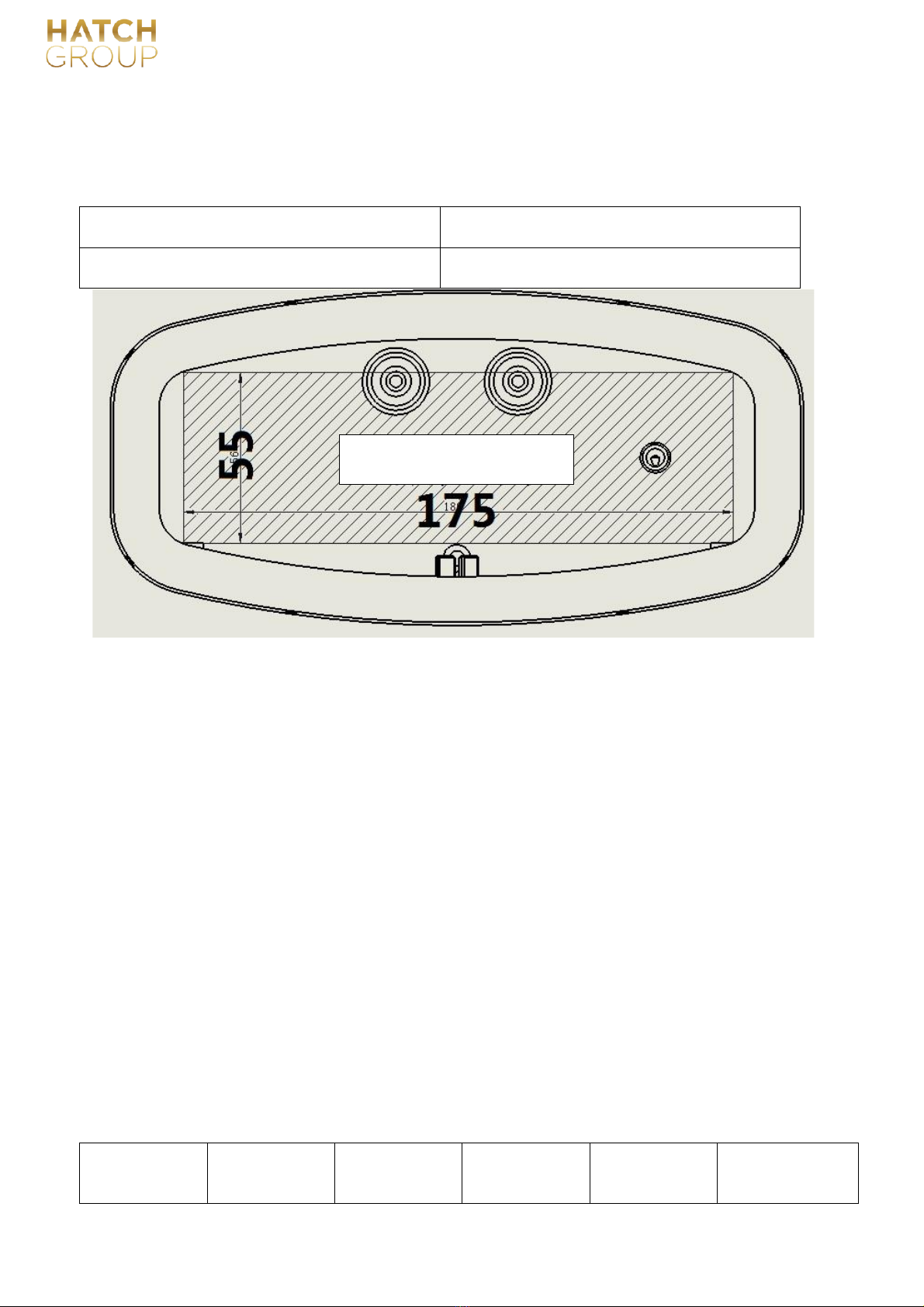
2.1 Chamber specification:
Available space size:
Model No.
Dimensions(DN * Length mm)
A50B
L=175, H=55, D=280
2.2 Parameter of Chamber:Design
pressure: -0.1/0.27MPa;
Design temperature:140℃
2.3 Parameter of autoclave:
Max working temperature:137℃
Max working pressure:0.24MPa
Safety valve set-pressure:0.24MPa;Safety valve open-pressure:0.24MPa~0.26MPa
Capacity of clean water tank:1L
Capacity of waste water tank:0.8L
Power input:220V±22V, 50Hz,2900VA
Life span:5 年
2.4 Environmental requirements for use: Environment
temperature: 5℃~40℃;
Relative humidity: ≤85%;
Ambient atmospheric pressure: 80kPa~106kPa;
Water usage requirements as shown in the table below:
Evaporation
residue
Silica
Iron
Cadmium
Lead
Other heavy
metals
Space available
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
10
≤10mg/L
≤1mg/L
≤0.2mg/L
≤0.005mg/L
≤0.05mg/L
≤0.1mg/L
Chloride
Phosphate
Conductivity
PH value
Conductivity
hardness
≤2mg/L
≤0.5mg/L
≤15uS/cm
5~7.5
Colorless clean
without
precipitation
≤0.02mmol/L
2.5 Transportation and storage conditions:
Ambient temperature: 5 ~ 40 ℃
Relative humidity: ≤80%
No corrosive gas
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
11
3. Installation
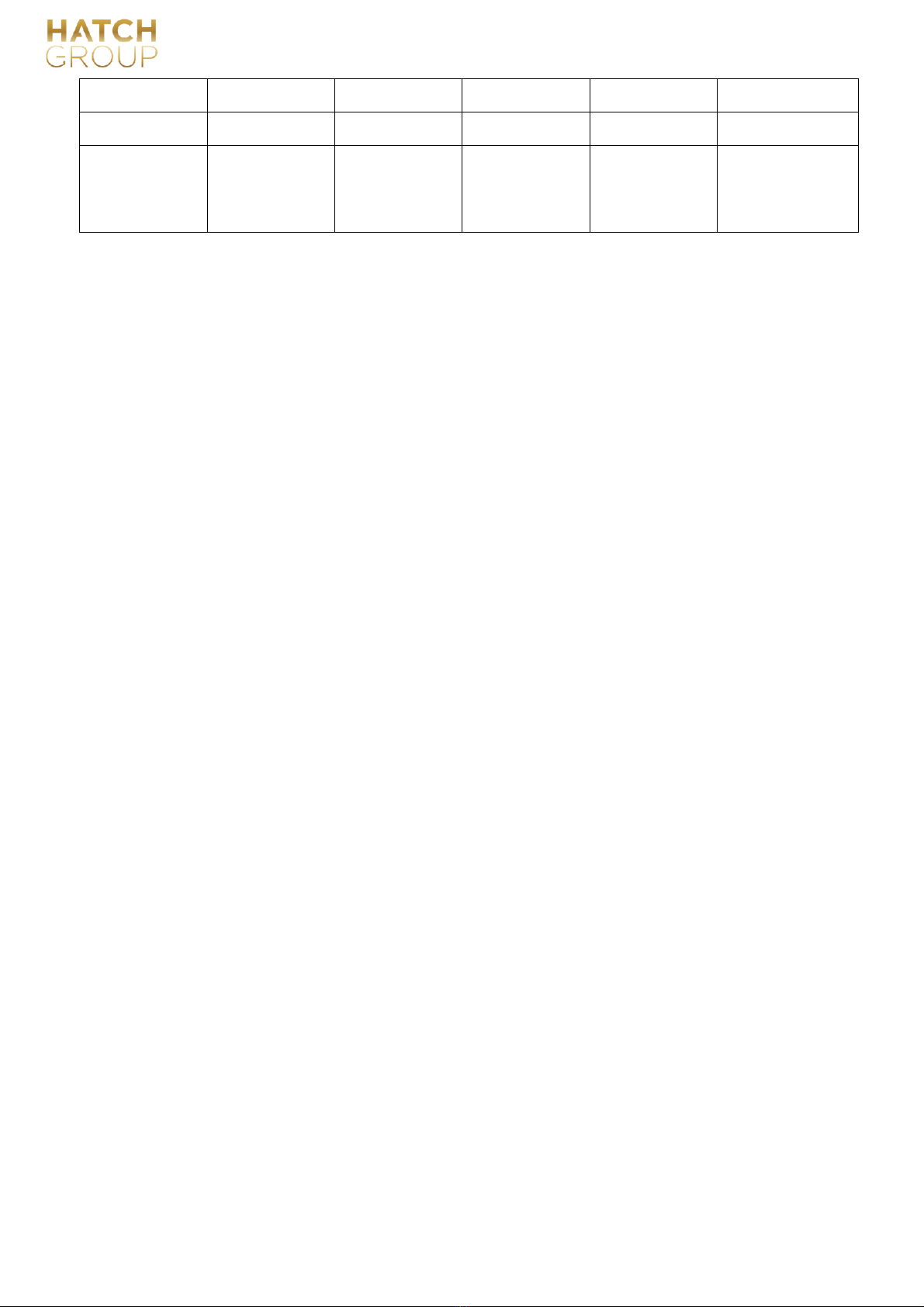
3.1 Product dimensions and weight
The external dimensions of A50B equipment are as follows (unit: mm),
Equipment weight:
Model
Gross weight (kg)
Net weight (9kg)
A50B
24
20
3.2 Installation requirement
In order to ensure the safe and reliable work of the equipment, please check whether it meets the
requirements according to the following clauses.
3.2.1 Power requirements: AC220V 50Hz unidirectional power supply, voltage fluctuation does
not exceed ± 10%, power> = 3.5kVA;
3.2.2 Environmental requirements: This equipment is required to be installed in a clean, dry,
well-ventilated indoor environment with small temperature difference. For temperature, humidity and
ambient atmospheric pressure requirements, see chapter 2.4.
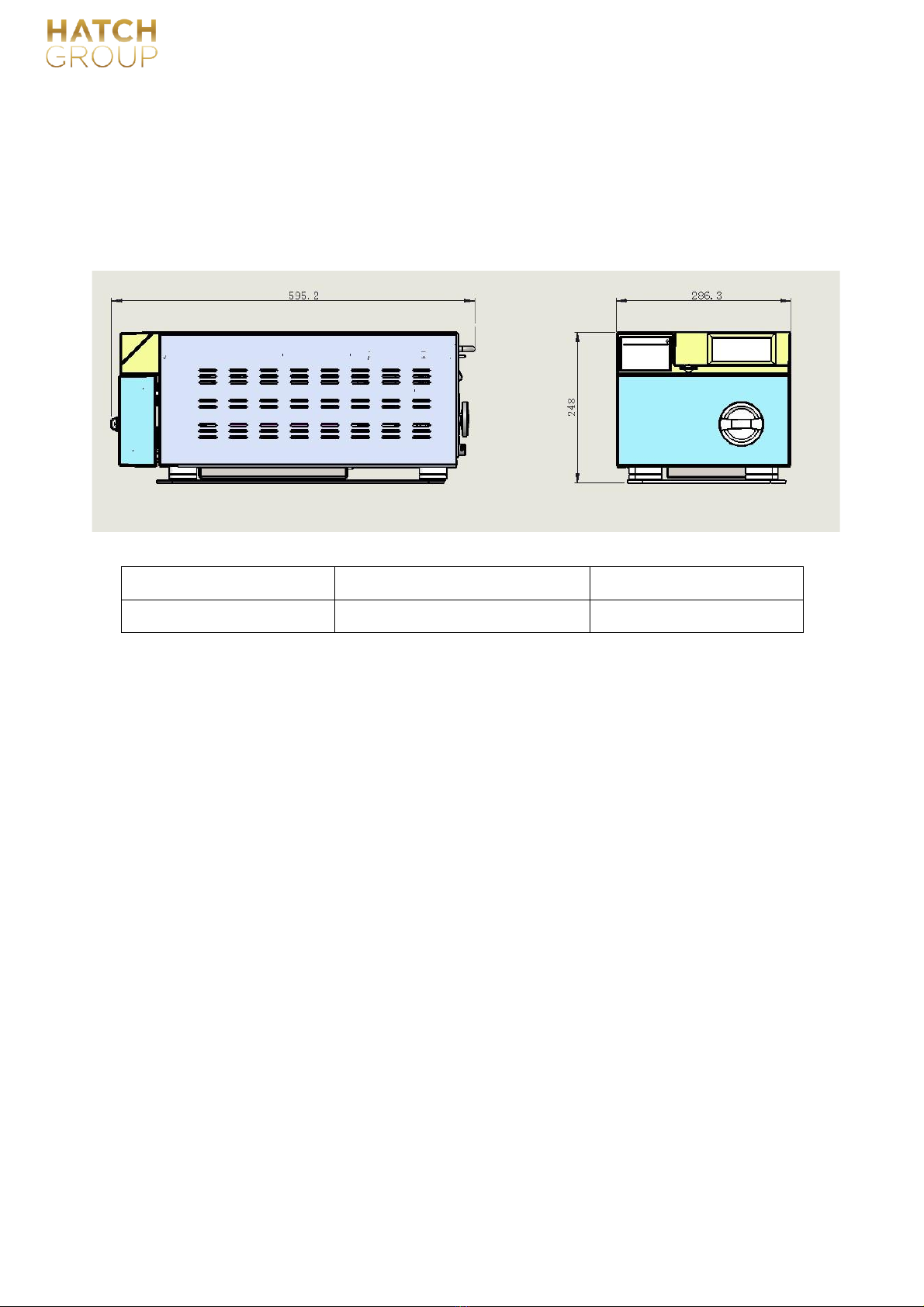
3.3.3 Installation space requirements: at least meet the minimum size requirements of the
following figure, see below.
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
12
Minimum width of table L1
Left side to wall L2
Minimum Height
55mm
≥100mm
0.7m
3.3.4 Installation table requirements: The installation table is strong enough to bear the weight
of the device and the sterilized object. The table can withstand at least 40kpa pressure.
Do not install the device in a closed cabinet; make sure that the shelf or table on which
the sterilizer is placed is strong enough; do not block the vent of the sterilizer.
3.3 Handling of packaged products
To ensure safe handling, please use a cart and similar tools to move the device. If there are no carts
or similar tools, at least two people are required to carry them in the front and back of the packing
box.
When handling by people, please pay special attention to avoid danger.
3.4 Unbox
After disconnecting the packing tape on the package with scissors, use a flat-blade screwdriver to
open the lock at the bottom of the box, remove the packaging cover, and finally remove the plastic
bag.
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
13
3.5 Lift the device onto the installation surface
The device can be placed by one person, after the device is lifted between the front and rear
feet of the product, it is placed on the workbench. Please note the following when handling:
It is strictly forbidden to lift the door during transportation to avoid damage to the
device;
Do not lift the foot pads of the device during handling to avoid personal injury;
Do not roll-proof when transporting, put the device on its side or upside down;
The recommended location for equipment handling is as follows:
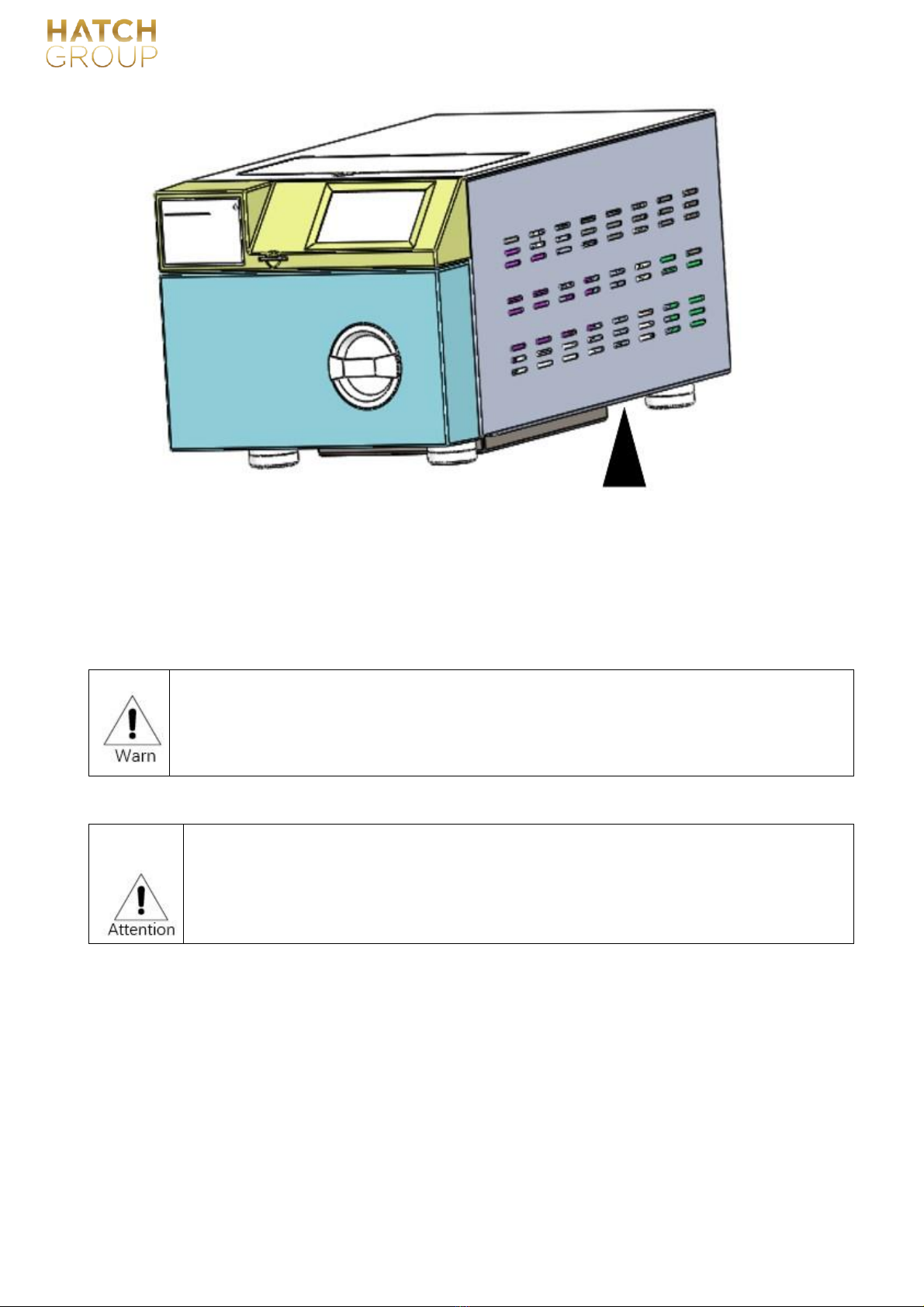
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
14
3.6 Feet adjustment
The height of the feet has been adjusted when the equipment leaves the factory. If the installation
surface is flat, no adjustment is needed; if the surface is uneven, the feet of the sterilizer should be
adjusted to make the sterilizer roughly flat.
3.7 Insert the power plug into the socket.
Make sure that the equipment is reliably grounded.
3.8 Other considerations
After the device has been transported and stored in an environment below 2 ° C. The
equipment must be placed in an environment with a temperature not lower than 5 ° C for
2 hours before operation. This kind of situation usually occurs in winter, and the freezing
of water in the equipment pipeline may cause equipment failure.
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
15
4. Adjusting
To ensure the normal use of the sterilizer, the sterilizer cannot be delivered for use until the adjusting
work is successfully completed. The specific debugging steps are as follows:
4.1 Atmospheric pressure resetting
After the device is installed in the first time or moved, the atmospheric pressure needs to be reset.
Otherwise, the door may not open (E10, E11).
Steps for resetting the atmospheric pressure:
Open the door, turn off the power, wait for 20s, turn on the power, wait for 1 minute.
4.2 Date and time setting
Check the date and time are correct, if not correct, make adjustment, see chapter 6.1.4.2 The date
and time have been set when the device leaves the factory, usually no adjustment is required。
4.3 Leak test
The leak test is done to ensure the tightness of the sterilization chamber and its pipelines to ensure
that the cold air in the sterilization chamber is eliminated. This device comes with a leak test program,
the program is "vacuum test". Run this program in the cold state. After the test, the device will display
the test results. Can only be used after passing this test
The test results may be inaccurate when the sterilization chamber is hot, and must be
tested when the sterilization chamber is cold.
4.4 Sterilization parameter confirmation
Use chemical indicator test for preliminary sterilization parameter confirmation
This test requires a B-D test package or PCD test device
Put the B-D test package or PCD test device in the sterilization room, and place it on the lower
floor near the door. Run the B-D test program. After the program is completed, remove the test
package and observe the indicator paper. The indicator paper changes color evenly to the required
color to be qualified.
5. Introduction of preset programs
5.1 Parameters of preset programs
Program
Sterilization
temperature
(℃))
Holding time
(Min)
Pre-vacuum
times
Drying time
(Min)
Remarks

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
16
5.2 Introduction to the load of each program
5.2.1 Load type corresponding to each program
134℃ universal
quick
Non-wrapped load or wrapped solid, fabric and cavity load, Paper-plastic packaging or
double-layer packaging may not dry, should be used immediately after sterilization
Universal
Non-wrapped load or wrapped solid, fabric and cavity load
134℃ solid
Solid load without package, should be used immediately after sterilization
B-D/Helix test
B-D test device or PCD test device
Vacuum test
Without any loading
Drying
Dry the load when it is not dry after sterilization
5.2.2 Applicable maximum load table
Model
Maximum weight of instruments
Maximum weight of fabric loads
A50B
2.2kg
0.5kg /package
5.3 Maximum working time and maximum water consumption when each program is at maximum
load
Model
Loading
kg
134℃
quick
Universal
134℃ Universal
121℃ Universal
134℃ Solid
Longes
t time
min
Min
water
consump
tion
mL
Longest
time
min
Min
water
consump
tion
mL
Longest
time min
Min
water
consump
tion
mL
Longest
time
min
Min
water
consump
tion
mL
134
℃
universal quick
134
Preset
4
3
4
℃
134
universal
Preset
134
3
6
4
4
134
℃ solid
134
1
2
121
℃
universal
121
Preset
8
3
4
B-D/Helix test
134
3.5
3
4
Test program
Vacuum test
Vacuum to -80kPa
,
holding 15min
Test program
Drying
5
min
Preset drying
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
17
LFSS0
3AA
2.2kg
20
280
24
300
38
380
18
240
6. User interface description
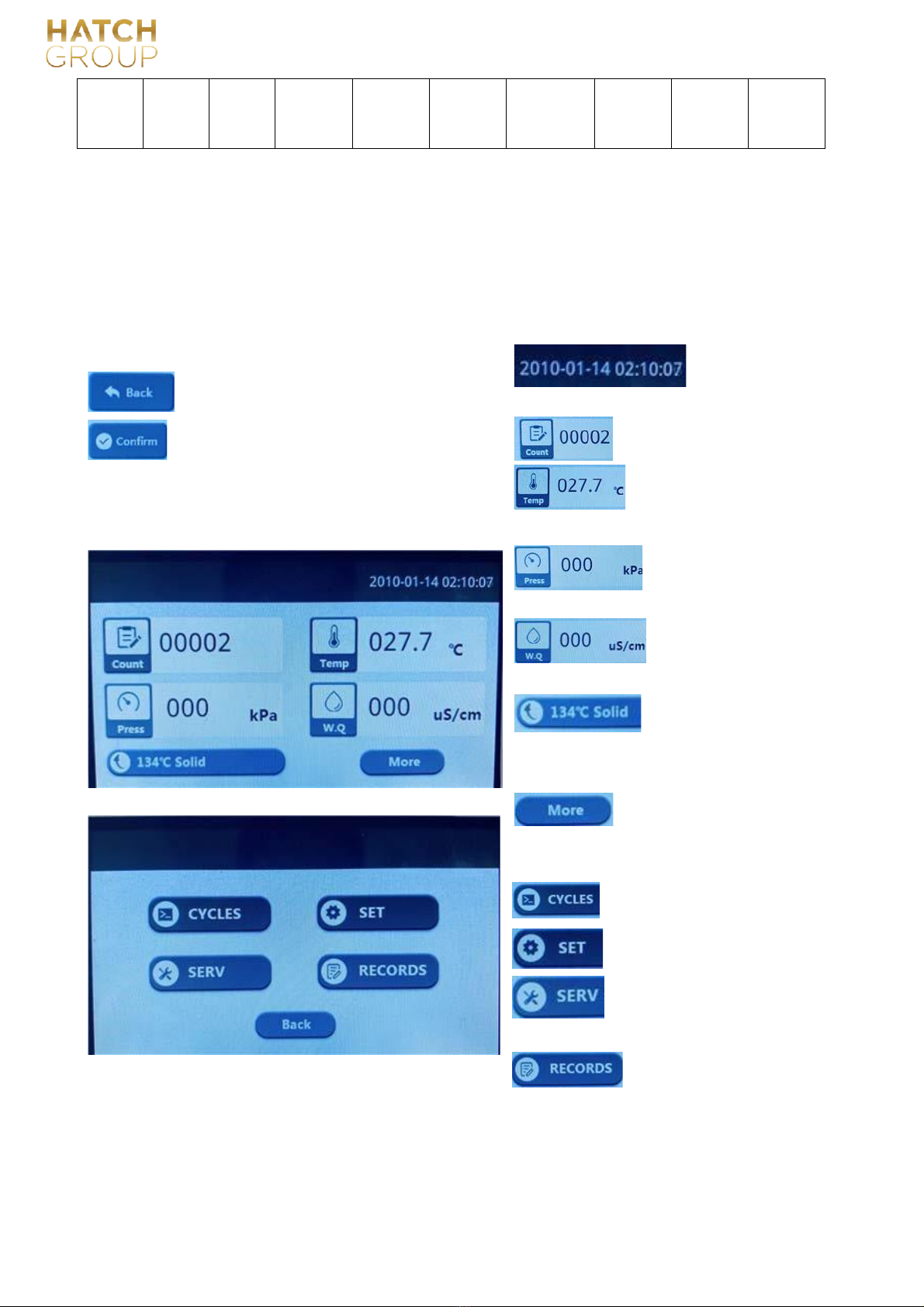
This device uses a 4.3-inch touch screen as a human-computer interaction interface.
6.1 Menu page introduction
Introduction of general keys:
: Display the current date
:Back to the previous interface;and time.
:Save Settings;: Show the current number of cycles;
6.1.2 Home Page : Display the current temperature
in the sterilization chamber;
: Display the current
pressure in
: Shows the quality of
the water
: Shortcut key, the
default is the
last run program, the
user can directly click
here to
:Click here to have
more options:
including program
menu, setting menu,
service
:Programs
for user to select;
:Settings for user to choose;
6.1.2
More Options
the sterilization chamber;
in the tank;
start this program;
menu and recording menu.
dealer;
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
18
:Service Menu, for manufacturer or
:for user, the check the latest 20
cycles, also available to print
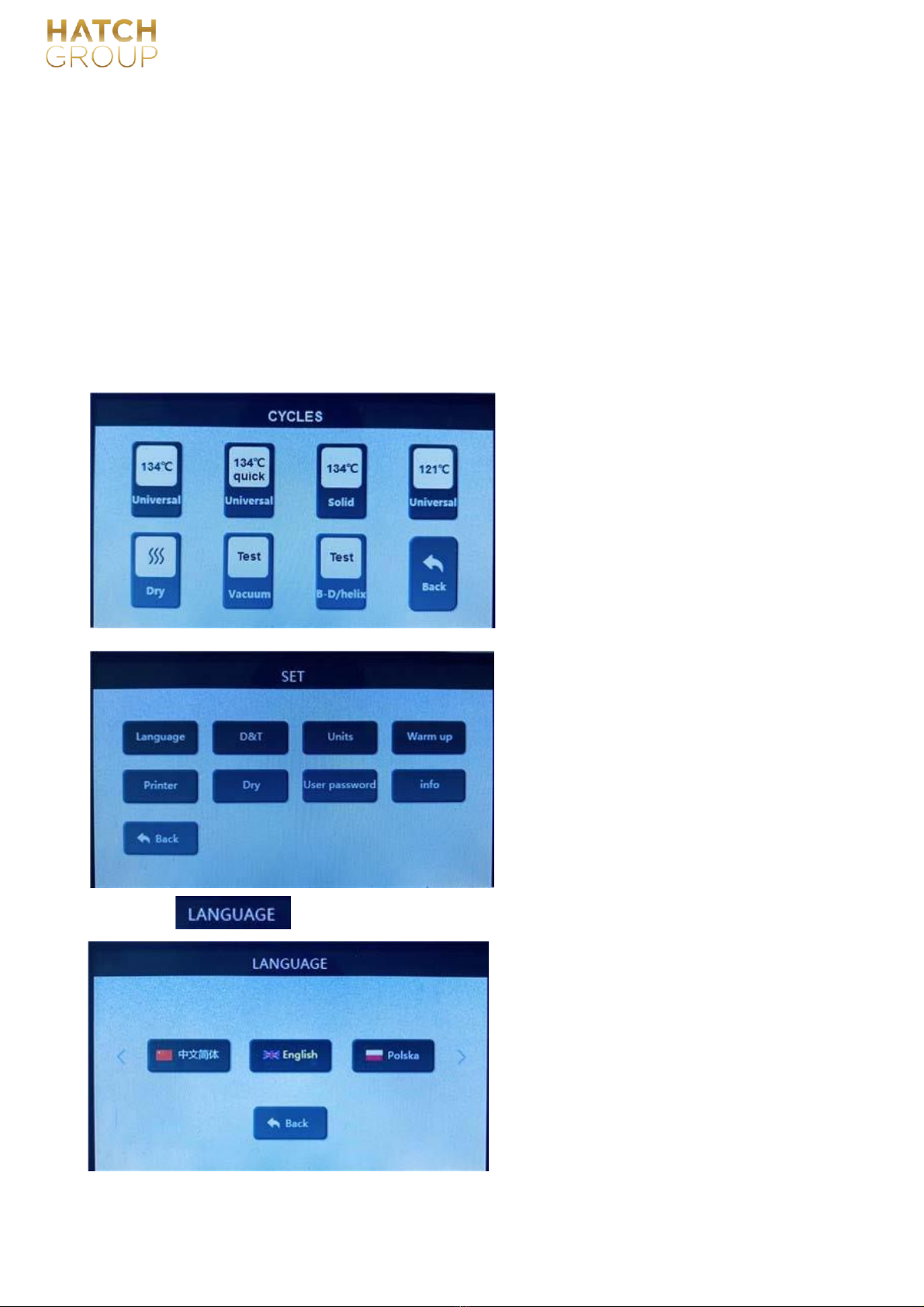
6.1.3 Programs (totally 7)
4 sterilization programs:134℃ unniversal,134℃ universal quick,134℃ solid,121℃ universal;1
dry program:dry
2 test programs:Vacuum test, to check the leakage of machine;
B-D/helix test, to check the effectiveness of program;
Remarks: For preset parameters of each program, corresponding load information and other information, please refer to
Chapter 5 of this manual.
6.1.4 Settings
Press
the corresponding button to select the desired language.
6.1.4.1
:
to select languages:

No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
19
6.1.4.2 :Used to set the date and time, after entering the page is as below:
The left picture shows that the current time is
13:11pm, Mar 30th 2020
YY:year
MM:month DD:day
hh: hour
mm:min
6.1.4.3 :Setting units for temperature and
pressure, the page after entering is like below:
The current unit is indicated in yellow font. Select the
corresponding unit and press OK to change the unit accordingly.
6.1.4.4 :The user sets whether the power-on preheating function is turned on or off. After entering, the
page is like this:
When set to "On": The equipment will start to warm
up and keep warm after being powered on, in order
to shorten the sterilization cycle time;
When set to "off": the device will not preheat after
power on, the device will only start to preheat after
the program is running,
the entire sterilization cycle time will be 5-7
minutes longer than the "on"
It is recommended to set this setting to "On".
When this setting is turned on,
special care should be taken not to touch the sterilization
chamber when the door is open to avoid burns.
No.:GC-JS-21 Version: 01/00 Effective date:2020-03-30
20
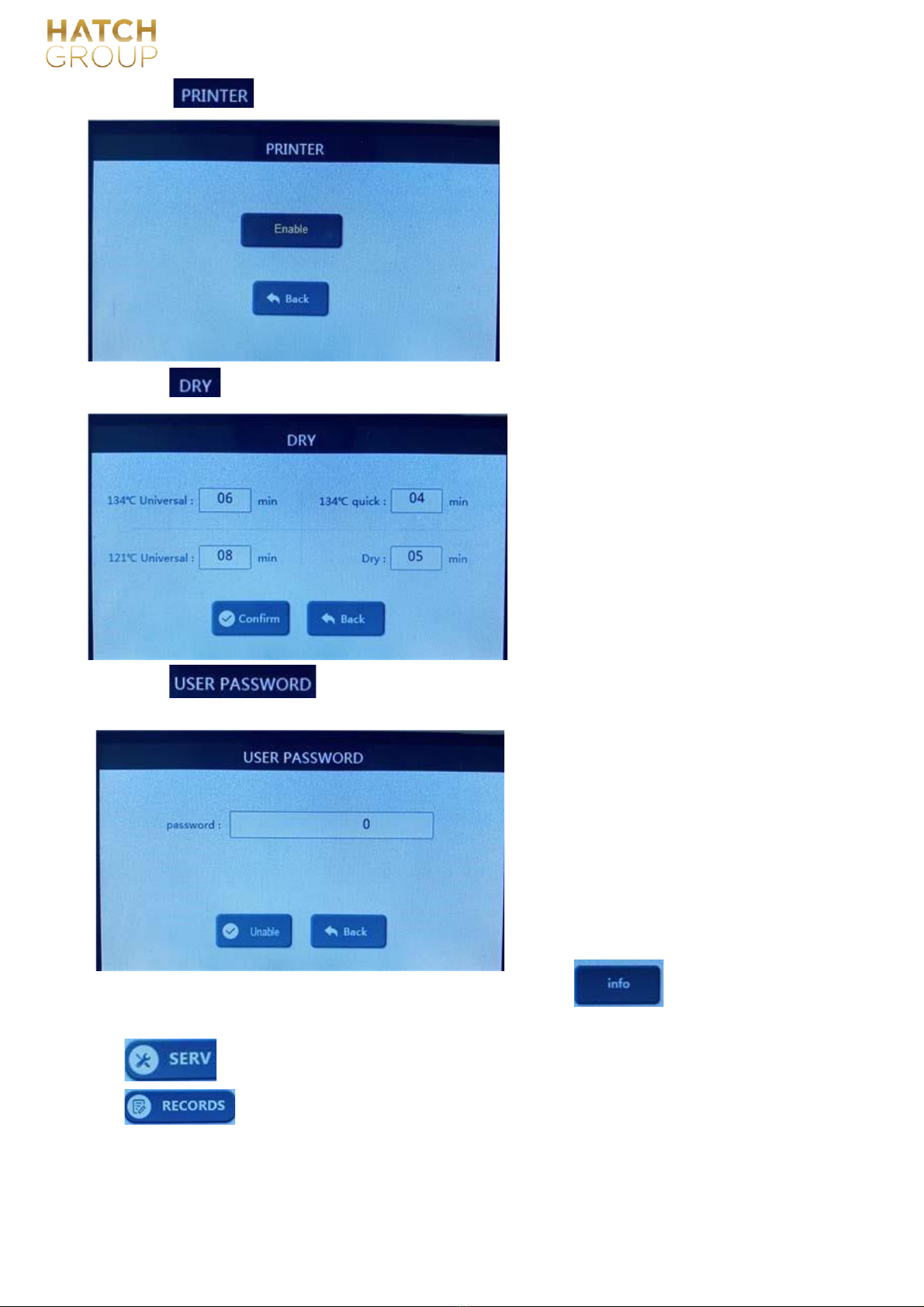
:The user sets whether to print records or not, and the
page after entering is like this:
On: printer prints records, SD card stores records;
Off: printer does not print records, SD card stores
records;
6.1.4.6 :The user can change or set the drying time of 4 programs.:
Adjustment range: 0 ~ 60 minutes.
Drying time of 134 ℃solid program is not
adjustable
If the user finds that the drying effect after
sterilization is not good, the user can increase
the drying time of the
corresponding program here.
The drying time can be changed according to the
time display.
6.1.4.7 :The user can set whether to enter with a password when starting up, and set the
entry password:
Press the "Enable" button to set whether this
function is enabled:
Enable: This function is turned on, and you
need to enter the password when you turn on
the computer to enter.
Not enabled: This function isnot turned on, and
it is directly entered when starting up.
Press the password display to change the
password.
6.1.4.8 :Enter here to view the
information of this device
6.1.5 Users cannot enter, only the manufacturer or the manufacturer's representative. Password required.
6.1.6 The user can enter to view and make up the sterilization record for nearly 20 times, see
7.12 for details.
6.1.7 To-be-run page and running interface
6.1.7.1 After the program is selected, enter the interface to be run, the interface is as follows
6.1.4.5
Table of contents
Popular Steriliser manuals by other brands

Market Forge Industries
Market Forge Industries STM-ED owner's manual

Air Cleaner
Air Cleaner profiSteril 100 user manual

Smeg
Smeg DRY50V instruction manual

Elecro Engineering
Elecro Engineering H.R.UV-C SPA-PRO Installation and operating manual

Gorenje
Gorenje K10BY instruction manual

Memmert
Memmert SM 200 operating instructions