Hikoneb 908 DC Configuration guide

Hikoneb 908 DC
User Manuel
Benutzerhandbuch
Manuel de l’Utilisateur
Istruzioni per l‘uso
Manual do Utilizador
Manual del usuario
EN
DE
FR
IT
PT
ES
• Large Ultrasonic Nebulizer
• Ultraschallvernebler
• Nébuliseur Ultrasonique
• Nebulizzatore Grande ad Ultrasuoni
•Nebulizador ultra-sónico de
grandes dimensões
• Nebulizador ultrasónico grande

LARGE
ULTRASONIC
NEBULIZER

1. Symbol Definitions 14
2. Important Safeguards 15-17
3. How the device works 17-18
4. Device Set-Up 18-20
5. Device Image and Components 20-21
6. Operating the device 22-23
7. Device Settings 23-25
8. Troubleshooting 26
9. Maintenance, Repair and Disposal 27-28
10.Cleaning, Disinfection, Sterilization 28-30
11. Accessories 30
12.Technical Specifications 31
13.Electromagnetic Conformity 32-34
Table of contents

ULTRASCHALLVERNEBLER

1. Symboldefinition 36
2. Wichtige Sicherheitshinweise 37-39
3. Das Gerät im Überblick 40-41
4. Einrichtung des Hikoneb 908 DC 41-42
5. Abbildung Hikoneb 908 DC und Komponenten 43-44
6. Inbetriebnahme des Hikoneb 908 DC 44-45
7. Geräteeinstellungen 46-49
8. Störungen/Fehlerbeseitigung 49-50
9. Wartung, Reparatur und Entsorgung 50-51
10.Reinigung, Desinfektion und Sterilisation 51-53
11. Zubehör 53-54
12.Technische Daten 54-55
13.Elektromagnetische Verträglichkeit 55-57
Inhaltsverzeichnis

NÉBULISEUR
ULTRASONIQUE

1. Définition des symboles 60
2. Mise en garde importante 61-63
3. Principe de fonctionnement du dispositif 63-65
4. Configuration du dispositif 65-66
5. Image et composants du dispositif 67-68
6. Utilisation du dispositif 68-69
7. Paramètres du dispositif 69-72
8. Dépannage 72-73
9. Entretien, réparation et élimination 73-74
10.Nettoyage, désinfection, stérilisation 75-76
11. Accessoires 77
12. Spécifications techniques 77-78
13.Conformité électromagnétique 79-81
Table des matières

NEBULIZZATORE
GRANDE AD
ULTRASUONI

1. Definizioni dei simboli 84
2. Importanti misure di sicurezza 85-87
3. Funzionamento del dispositivo 87-88
4. Configurazione del dispositivo 89-90
5. Immagine e componenti del dispositivo 90-91
6. Funzionamento del dispositivo 92
7. Impostazioni del dispositivo 93-96
8. Risoluzione dei problemi 96-97
9. Manutenzione, riparazione e smaltimento 97-98
10.Pulizia, disinfezione e sterilizzazione 98-100
11. Accessori 100
12.Specifiche tecniche 101
13.Conformità elettromagnetica 102-104
Indice dei contenuti

NEBULIZADOR
ULTRA-SÓNICO DE
GRANDES DIMENSÕES

1. Definições de Símbolo 106
2. Salvaguardas importantes 107-109
3. Como funciona o dispositivo 109-110
4. Configuração do dispositivo 111-112
5. Imagem e Componentes do Dispositivo 112-113
6. Funcionamento do dispositivo 114
7. Configurações do dispositivo 115-118
8. Resolução de problemas 118
9. Manutenção, reparação e eliminação 119-120
10.Limpeza, Desinfecção, Esterilização 120-122
11. Acessórios 122
12.Especificações técnicas 123
13.Conformidade electromagnética 124-126
Índice

NEBULIZADOR
ULTRASÓNICO GRANDE

1. Definiciones de símbolos 128
2. Salvaguardias importantes 129-131
3. Funcionamiento del dispositivo 131-132
4. Configuración del dispositivo 133-134
5. Imagen del dispositivo y componentes 134-135
6. Funcionamiento del dispositivo 136-137
7. Configuración del dispositivo 137-140
8. Solución de problemas 140-141
9. Mantenimiento, reparación y eliminación 141-142
10.Limpieza, desinfección y esterilización 142-144
11. Accesorios 144-145
12.Especificaciones técnicas 145-146
13.Conformidad electromagnética 146-148
Indice
Hikoneb | 908 DC
14
User Manual
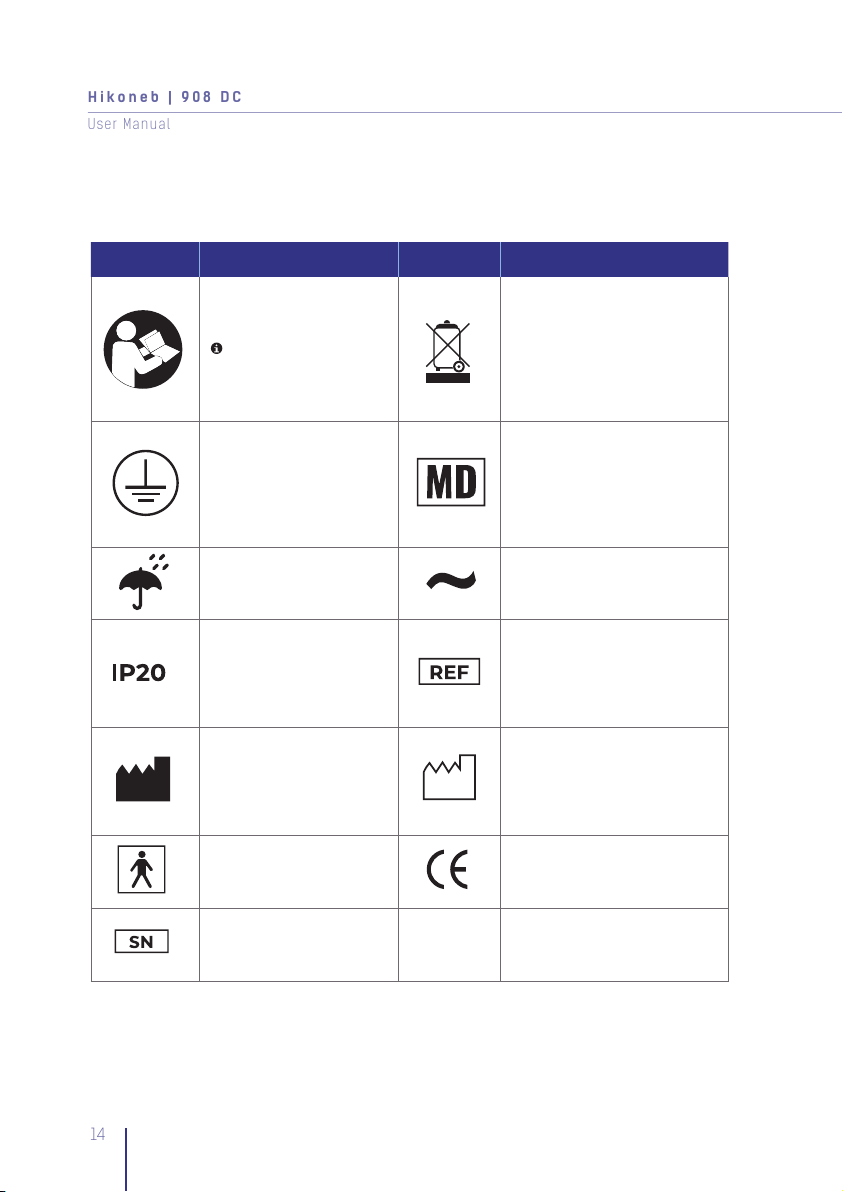
1. Symbol Definitions
It is mandatory to read and
understand the operating
instructions prior to use.
This symbol has a blue
background on the product
label
This device contains electronical
and/or electrical equipment
that must be recycled per EU
Directive 2012/19 EU-Waste
Electrical and Electronic
Equipment (WEEE)
Type of Protection. Ground
Conductor as per Class I Medical device
Keep dry IAC Voltage
The device is protected
against solid objects of
12.5mm and more Item code
Manufacturer Date of production
Type B applied part European CE-Mark
Serial Number

15
Hikoneb | 908 DC
User Manual
To reduce the risk of burns, electrocution, fire, or injury to persons:
1. The device should never be left unattended when plugged in. Close supervision is
necessary when this product is used for or on near children or physically incapacitated
individuals.
2. Use this product only for its intended use as described in this guide. Only use
attachments/spare parts recommended by the manufacturer.
3. Do not nebulize home-therapy or aroma-therapy oils with this device.
4. Do not use on ventilated patients.
Never operate this product if:
1. It has a damaged power cord or plug.
2. It is not working properly.
3. It has been dropped or damaged.
4. It has been dropped into water.
2. Important Safeguards
When using electrical products, especially when children are present, basic safety
precautions should always be followed.
Read all instructions before using. Important information is highlighted by
these terms:
DANGER – Urgent safety information for hazards that will cause serious injury or death.
WARNING – Important safety information for hazards that might cause serious injury.
CAUTION – Information for preventing damage to the product.
NOTE – Information to which you should pay special attention.
To reduce the risk of electrocution:
1. Always unplug this product immediately after using.
2. Do not use while bathing.
3. Do not place or store product where it can fall or be pulled into a bath tub or sink.
4. Do not place in or drop into water or other liquid.
DANGER!
WARNING!

Hikoneb | 908 DC
16
User Manual
Return the product to an authorized Drive DeVilbiss service centre for a technical
inspection and repair.
• Keep the power cord away from heated surfaces.
• Never drop or insert any object into any opening or tube.
• Do not use outdoors. This device is designed to be used inside.
• Do not use in an oxygen enriched environment.
• Connect this product to an appropriate electrical outlet only.
• Do not use this product with a DC to AC converter, or with any AC voltage and/or
frequency other than specified.
• The Hikoneb 908 complies with EMC requirements conforming to IEC:60601-1-2.
Nevertheless, location should be considered with caution, when this product is used in
the vicinity of other electronic equipment where electromagnetic interference may be
an issue.
• Make sure that all parts are connected correctly, and that the dust filter is clean.
• A filter which has changed its colour or used a period of 3 months, should be replaced.
• Bacteria filter should be replaced every 48 hours, in between patients and when it
gets dirty.
• Medicine cups (38) are for single use only.
• Dispose of any remaining medicines. Do not use again.
• The device has been designed for inhalation using a mouthpiece or a face mask.
When only using the aerosol tube (for humidification purposes), there should be at least
10cm distance between a patient and the tube.
• Change mouthpiece daily or when changing patient.
• Room/ambient temperature should not exceed 30°C when used with the heater
system (19).
• Only medical clean/sterile water should be used for nebulization.
• Medicines can be nebulized as aqueous solution only.
• The nebulizer chamber (11) is equipped with a maximum and minimum water level
mark. Water should be filled into the chamber between these two marks. If a sterile
water bottle is used, the gasket should be placed completely on the bottle lid, to
prevent flowing water from the chamber. In addition, make sure that the lid is tightly
WARNING!
NOTE!

17
Hikoneb | 908 DC
User Manual
secured on the sterile water bottle.
• After each use, this system should be cleaned, disinfected, and sterilized.
• The device should be used with original manufacturers air filter (23).
• Caution should be taken when removing the chamber, tubing/tube heater after
therapy, as these items may be very warm.
• Only authorized service personnel are allowed to open the device to conduct
troubleshooting and failure analysis. Do not try to open or repair the device by yourself.
• Use original parts and accessories only.
The Hikoneb 908 uses an ultrasonic frequency to turn a liquid medication into an
aerosol.
The device can be started and controlled by using push buttons. The base of the
nebulizer chamber contains a transducer, which causes the liquid in the chamber
to nebulize through its high-frequency oscillations. A blower located in the housing
conveys the aerosol through the patient tube to the patient for inhalation. This
driving air is passed through a bacteria filter before nebulization.
The Hikoneb 908 is equipped with an alarm system. An audible alarm is activated
when water in the nebulization chamber is below the minimum water level and the
device will automatically switch off.
Service Life
The expected service life of the device is 10 years.
Intended Use / Indication
The Hikoneb 908 DC has been manufactured for Ultrasonic Nebulizer Aerosol
treatment. It breaks water or liquid medicine into particles and produced
respirable water or medicine steam It is used for Asthma, Bronchitis, Pharyngitis
and Allergies and other respiratory diseases.
The Hikoneb 908 DC is used in hospital environments, hospital employees make
usage and maintenance operation. It is not suitable to connect to anesthesia
respiration system or ventilator systems. A doctor decides medicine type and
3. How the device works

Hikoneb | 908 DC
18
User Manual
usage, a user cannot practice this treatment directly by himself/herself. The
devices are not support, patient monitoring and sterilized devices. The device has
no measurement function.
Even though high-volume ultrasonic nebulizers are not intended for direct patient
application, mouthpieces and aerosol masks can be used when administering
medications and substances intended to be inhaled (antibiotics, broncho-dilators
and for sputum induction)
Not suitable to be used with respiration masks. This warning is to avoid using with
protective masks such as N95. If a mask is to be used, then use aerosol/nebulizer
masks only.
Device intended use is for humidification of patient’s respiratory air. Room
humidification for medical and non-medical applications use is prohibited.
Manufacturer is not liable for product failure, all kind of damages and accidents
caused by non-intended use.
Contraindications
- Coughing, bronchospasm or allergic reactions.
- Patients unable to spontaneously breathe.
- Inability of the patient to cooperate accordingly.
Target Group
Spontaneously breathing patients from the age of 16 years for aerosol therapy.
4. Device Set-up
Assembly Instructions
1. Assemble the 5 feet mobile stand by using a 6mm Allen key (included) to connect
the five wheels and five feet. (26 & 1) with the black middle base connector (3) and
6mm Allen bolts. Screw together the main stand rods (6) and connect to the base with
a 6mm bolt and Allen key.
2. Connect the main unit bracket clamp (5) with the assembled stand rod (6) by its
clamp and Allen key. Connect three knurled plastic locking nuts (4) below the device,

19
Hikoneb | 908 DC
User Manual
BACK SIDE OF THE DEVICE
Heater Socket 30
Main Fuse 32
Power Input 31
Air Filter 33
to the device´s (5). The distance between the device and the ground should not exceed
60cm; a higher position can affect stability of the assembly.
3. Clip and fix the bottle holder (8) to the stand (6) with its screw.
4. If entraining oxygen, connect the oxygen adapter to device outlet, then connect the
bacteria filter (23) to the oxygen adapter (21) and connect the incoming oxygen to O2
adapter. Oxygen adapter must always be connected before the bacterial filter (between
device outlet and bacterial filter). If connected between bacterial filter and ventilation
tube, oxygen will enter to the system without any bacterial filtration.
5. If heated nebulization is desired, then place the heater (19) on chamber between the
lid and the aerosol tubing (15) and connect its power plug to the socket on the back of
the device.

Hikoneb | 908 DC
20
User Manual
6. Attach one end of the ventilation tube (16) to the bacteria filter (23) and the other end
to the nebulization chamber lid.
7. Connect the adjustable arm set (14) to the main stand rod. Connect the aerosol tube
(15) to the arm set’s black plastic clips. The support arm should tighten and secured
ensuring that the position of the aerosol tube is pointing upwards and straight, allowing
any collected aerosol to flow back into the chamber.
8. To start the aerosol therapy, place the required dose of medication into the
disposable medication cup (38), and connect to the chamber (11) and its lid (36). Locate
and lock the nebulization chamber in its place to the unit (22) by turning to the right.
1. Wheel
3. Middle base connector
4. Locking nuts
5. Bracket clamp
6. Stand rod
8. Bottle holder
11. Nebuliser chamber
14. Adjustable arm set
15. Aerosol tube
16. Ventilation tube
19. Heater
21. Oxygen adapter
22. Holding device
23. Bacterial filter (round)
26. Five feet
36. Nebuliser chamber lid
38. Medical cup
5. Device Image and Components
NOTE-
The distance between device and ground should not exceed 60 cm.
Other manuals for 908 DC
1
Table of contents
Popular Medical Equipment manuals by other brands
biodex
biodex 058-720 Installation & operation manual

ResMed
ResMed S9 AutoSet CS-A Information guide

Gima
Gima 25820 Use and maintenance book

HEBU medical
HEBU medical ECO Accu HB 8870 Operating and service manual
Stryker
Stryker System 6 6205-000-000 Instructions for use

Canon
Canon OMNERA 400T Planning guide