32
HOW TENS WORKS
There is nothing “magic” about Transcutaneous Electrical Nerve
Stimulation (TENS). TENS is intended to be used to relieve pain.
The TENS unit sends comfortable impulses through the skin that
stimulate the nerve (or nerves) in the treatment area. In many cas-
es, this stimulation will greatly reduce or eliminate the pain sensa-
tion the patient feels. Pain relief varies by individual patient, mode
selected for therapy, and the type of pain. In many patients, the
reduction or elimination of pain lasts longer than the actual period of
stimulation (sometimes as much as three to four times longer). In
others, pain is only modied while stimulation actually occurs. You
may discuss this with your physician or therapist.
EXPLANATION OF EMS
Electrical Muscle Stimulation is an accepted and proven way of
treating muscular injuries. It works by sending electronic pulses to
the muscle needing treatment; this causes the muscle to contract.
It is derived from the square waveform, originally invented by John
Faraday in 1831. It works by directly stimulating motor neurons
which causes muscle contraction. It is widely used in hospitals and
sports clinics for the treatment of muscular injuries and for the re-
education of paralyzed muscles, to prevent atrophy in affected mus-
cles and improve muscle tone and blood circulation.
HOW EMS WORKS
1. Relaxation of muscle spasms
2. Prevention or retardation of disuse atrophy
3. Increasing local blood circulation
4. Muscle re-education
5. Immediate post-surgical stimulation of calf muscles to prevent
venous thrombosis
6. Maintaining or increasing range of motion
The EMS units send comfortable impulses through the skin that
stimulate the nerves in the treatment area. When the muscle re-
ceives this signal it contracts. As the signal strength increases,
the muscle contracts as in physical exercise. Then when the pulse
ceases, the muscle relaxes and the cycle starts over again, (Stimu-
lation, Contraction and Relaxation.) Powered muscle stimulators
Chapter 1: GENERAL DESCRIPTION
The Premier Combo Plus TENS/EMS is a fully digital battery oper-
ated pulse generator that sends electrical impulses to the nerves
and underlying muscle groups. This unit is a combination stimulator
of TENS and EMS which can be used for pain relief and muscle
stimulation. The device is provided with two controllable output
channels, each independent of the other. A pair of electrodes can
be connected to each output channel. The intensity level and set-
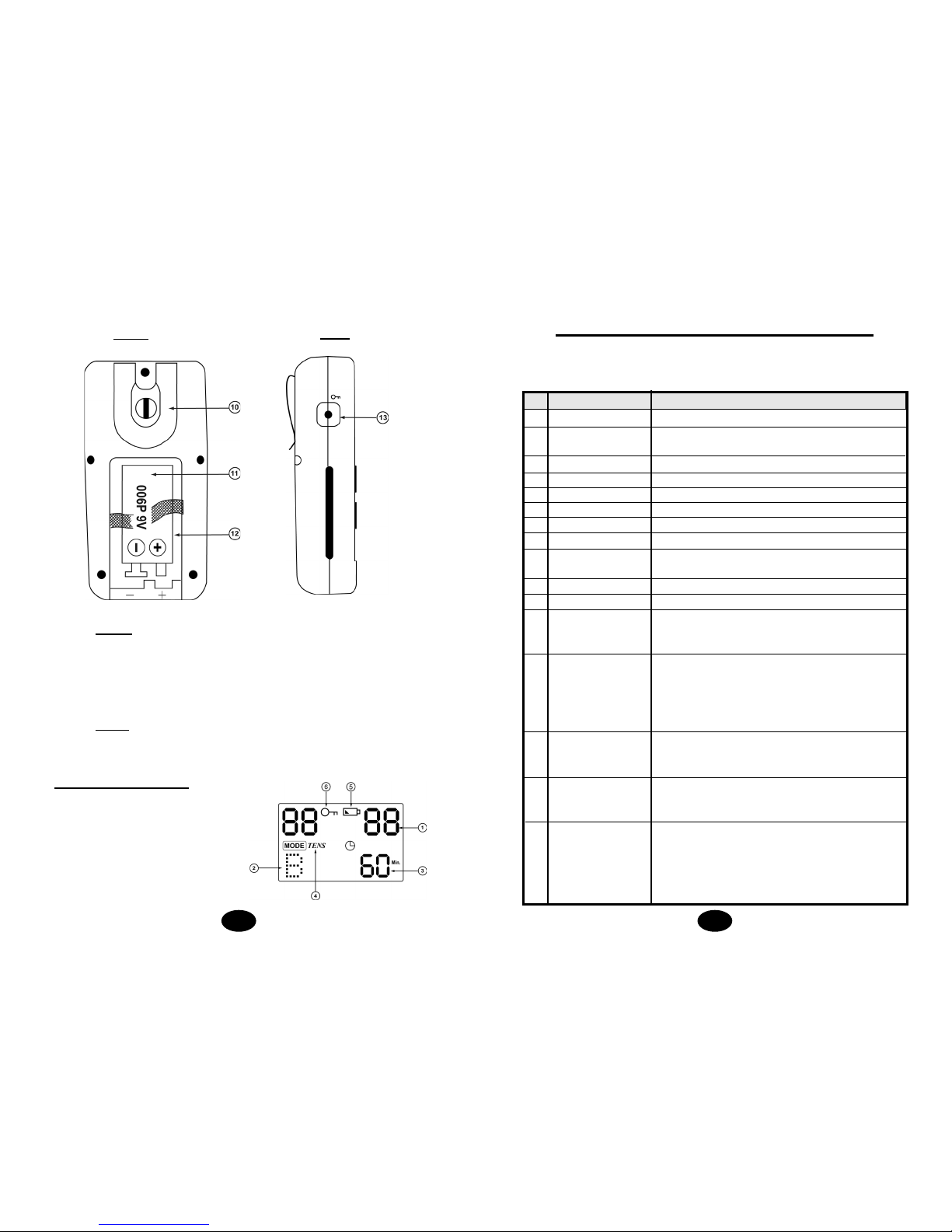
tings are controlled by press buttons
Chapter 2 : INTRODUCTION
EXPLANATION OF PAIN
Pain is a warning system and the body’s method of telling us that
something is wrong. Pain is important; without it abnormal condi-
tions may go undetected, causing damage or injury to vital parts of
our bodies.
Even though pain is a necessary warning signal of trauma or mal-
function in the body, nature may have gone too far in its design.
Aside from its value in diagnosis, long-lasting persistent pain serves
no useful purpose. Pain does not begin until coded messages
travel to the brain where they are decoded, analyzed, and reacted
to. The pain message travels from the injured area along the small
nerves leading to the spinal cord. Here the message is switched to
different nerves that travel up the spinal cord to the brain. The pain
message is interpreted and pain is perceived.
EXPLANATION OF TENS
Transcutaneous Electrical Nerve Stimulation is a non-invasive, drug-
free method of controlling pain. TENS uses tiny electrical impulses
sent through the skin to nerves to modify your pain perception.
TENS does not cure any physiological problem; it only helps control
the pain. TENS does not work for everyone; however, in most pa-
tients it is effective in reducing or eliminating the pain, allowing for a
return to normal activity.