Masimo EMMA User manual
Other Masimo Measuring Instrument manuals

Masimo
Masimo NomoLine ISA CO2 User manual

Masimo
Masimo Root RDS-7 User manual

Masimo
Masimo Opioid Halo User manual
Masimo
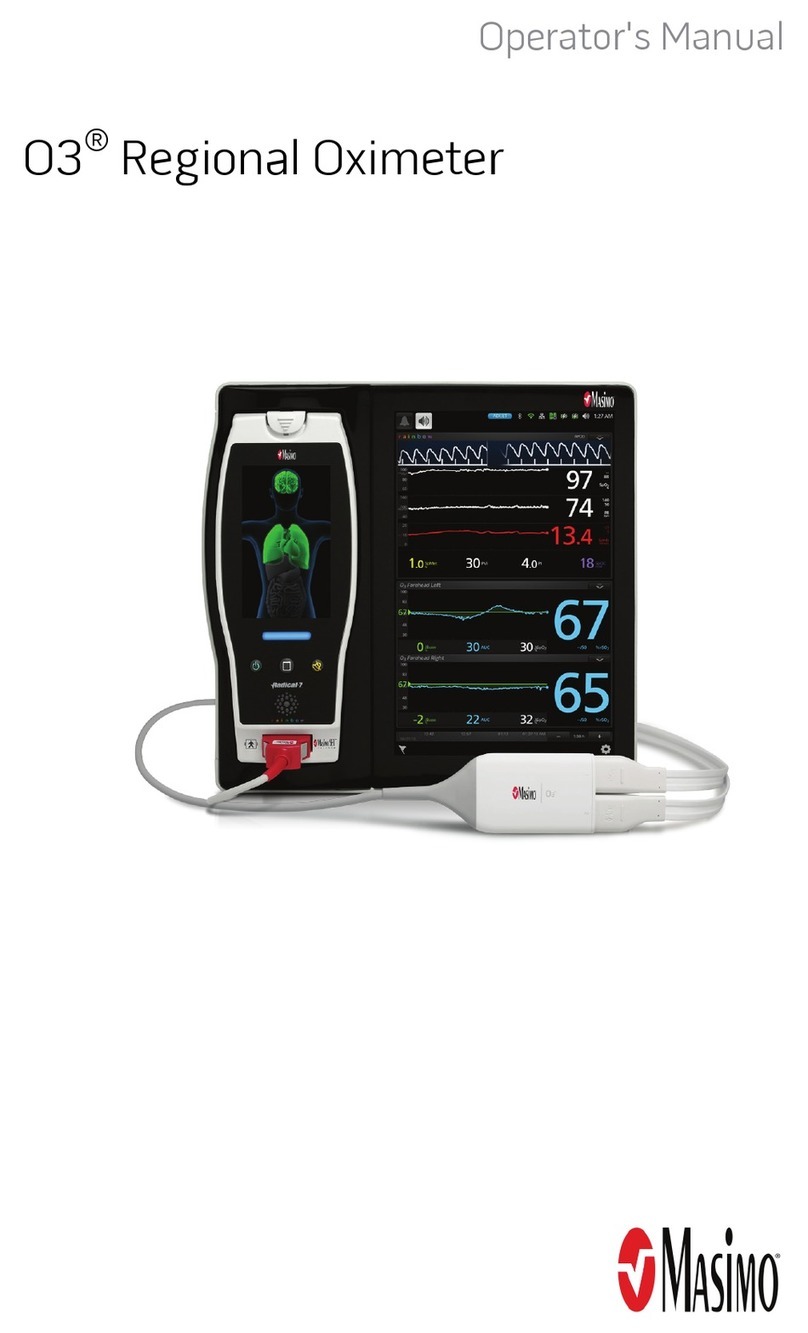
Masimo O3 Regional Oximeter User manual

Masimo
Masimo RAD-57C User manual

Masimo
Masimo Root RDS7A User manual

Masimo
Masimo EMMA User manual

Masimo
Masimo PRONTO-7 User manual

Masimo
Masimo Root User manual

Masimo
Masimo MightySat Rx User manual
Popular Measuring Instrument manuals by other brands

Powerfix Profi
Powerfix Profi 278296 Operation and safety notes

Test Equipment Depot
Test Equipment Depot GVT-427B user manual

Fieldpiece
Fieldpiece ACH Operator's manual

FLYSURFER
FLYSURFER VIRON3 user manual

GMW
GMW TG uni 1 operating manual

Downeaster
Downeaster Wind & Weather Medallion Series instruction manual

Hanna Instruments
Hanna Instruments HI96725C instruction manual

Nokeval
Nokeval KMR260 quick guide

HOKUYO AUTOMATIC
HOKUYO AUTOMATIC UBG-05LN instruction manual

Fluke
Fluke 96000 Series Operator's manual

Test Products International
Test Products International SP565 user manual

General Sleep
General Sleep Zmachine Insight+ DT-200 Service manual















