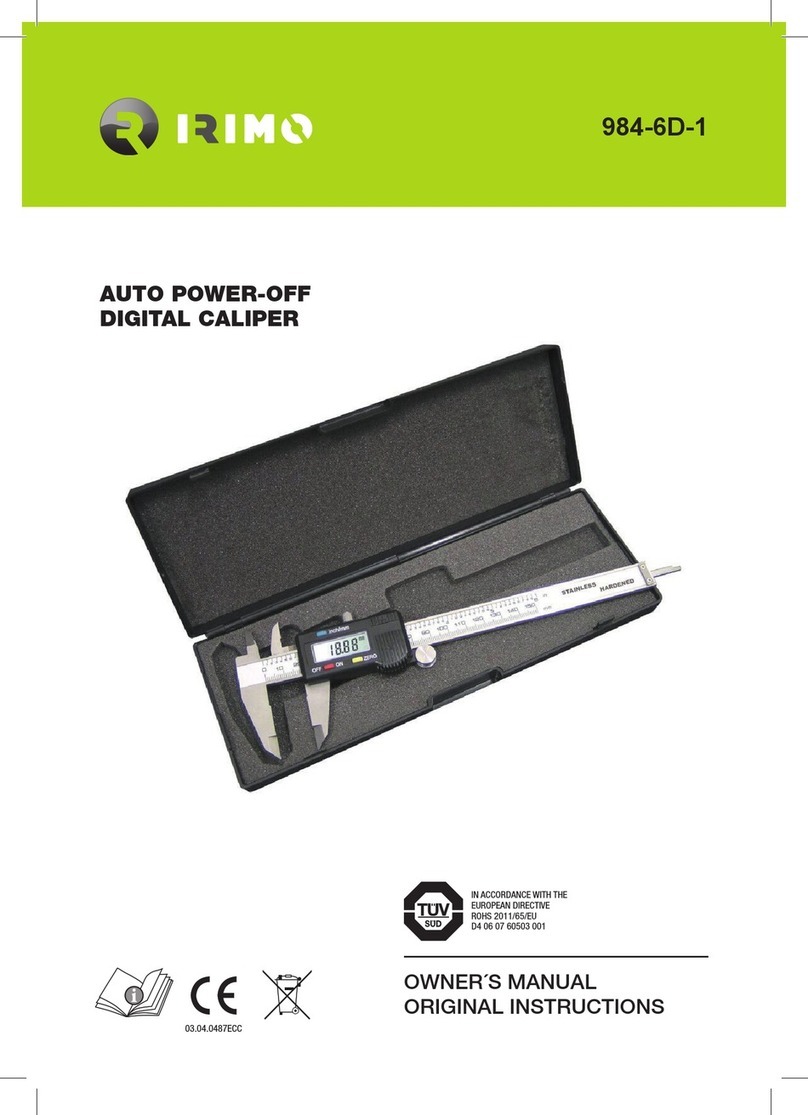
General Sleep Zmachine Insight+ DT-200 User manual

Copyright © 2018 by General Sleep Corporation
All rights reserved
Zmachine
®
Insight & Insight+
Model: DT-200
Clinician Instruction and Service Manual
Rev. 1.7


Clinician Instruction and Service Manual i
About this Manual
You are advised to read and understand this manual before using the Zmachine.
This manual contains all of the information that is needed to set up and operate the Zmachine, and
does not assume prior knowledge or experience with operator-programmable medical electronics
devices. Retain this manual for future reference.
The information in this manual has been carefully checked and is believed to be accurate.
However, in the interest of continued product development, General Sleep Corporation ("GSC")
reserves the right to make changes and improvements to this manual and to the product(s) that it
describes, at any time, and without notice or obligation.
Caution: Federal Law (USA) restricts this device to sale by, or on the order of, a physician
or other qualified healthcare practitioner licensed by the law of the state in
which he or she practices to use or order the use of this device.
Mailing and Shipping Address General Sleep Corporation
26250 Euclid Avenue, Suite 709
Euclid, OH 44132, USA
Telephone (888) 330-4424
Web Site www.GeneralSleep.com
Note: This manual is applicable to the Zmachine Insight & Insight+ with firmware
version 4.10.0 and later.

ii Clinician Instruction and Service Manual
Table of Contents
Introduction ................................................................................................................................... 1
Description ................................................................................................................................................ 1
Indications for Use ..................................................................................................................................... 1
The Zmachine at a Glance ......................................................................................................................... 2
Description of Reusable Components ........................................................................................................ 3
Description of Disposable Components .................................................................................................... 4
Description of Documentation and Software Components ........................................................................ 5
Glossary of Symbols .................................................................................................................................. 6
Contraindications, Warnings and Cautions ................................................................................ 7
Contraindications ....................................................................................................................................... 7
Warnings.................................................................................................................................................... 7
Cautions ..................................................................................................................................................... 8
Care and Maintenance ................................................................................................................ 10
Inspection ................................................................................................................................................ 10
Cleaning ................................................................................................................................................... 10
Interference .............................................................................................................................................. 10
Environmental Parameters for Shipping and Storage .............................................................................. 11
Environmental Parameters for Operation ................................................................................................ 11
Disposal of Equipment ............................................................................................................................ 11
Attaching the Hanging Cover .................................................................................................................. 11
Usage Overview ........................................................................................................................... 12
Understanding the Zmachine ................................................................................................................... 12
Zmachine New Patient Setup ................................................................................................................... 13
Pack Supplies for Patient to Take Home ................................................................................................. 13
Familiarize Patient with the Zmachine .................................................................................................... 13
Generate a sleep report and review .......................................................................................................... 13
Adjust System Setup & Discuss .............................................................................................................. 14
Re-Fill Zmachine Travel Case and Provide to Patient ............................................................................. 14
General System Setup and Operation ...................................................................................... 15
Charging the Zmachine ........................................................................................................................... 15
Booting the Zmachine ............................................................................................................................. 16
Accessing the Zmachine System Menu ................................................................................................... 17
Setting the Time and Date ....................................................................................................................... 18
Prepare the Zmachine for a New Patient ................................................................................................. 19
Setting the Alerts to Get-Out-Of-Bed (Insight+ only) ............................................................................. 20
Setting the Statistics to Display (Insight+ only) ...................................................................................... 21
Setting the Day Break Time .................................................................................................................... 22
Sleep Report and Statistics Files ................................................................................................ 23
Generate Sleep Report and Statistics ....................................................................................................... 23
MicroSD Card Operations (Eject, Copy Data, Insert) ............................................................................. 24
About the Sleep Report & Statistics Files ............................................................................................... 25
File: score.csv .......................................................................................................................................... 25
File: Stats.csv ........................................................................................................................................... 26
System Utilities ............................................................................................................................ 29
Display System Information .................................................................................................................... 29
Update Firmware ..................................................................................................................................... 30
Setting the Failed Sensor Alert ................................................................................................................ 31

Clinician Instruction and Service Manual iii
Troubleshooting and Specifications ........................................................................................... 32
Troubleshooting ....................................................................................................................................... 32
Zmachine Insight & Insight+ Specifications ........................................................................................... 33
Clinical Performance .................................................................................................................. 34
Clinical Performance Summary ............................................................................................................... 34
Legal Notices ................................................................................................................................ 35
Trademark and Intellectual Property Rights ............................................................................................ 35


Introduction 1
1
Introduction
Description
The Zmachine is a high technology sleep monitor, developed by General Sleep Corporation,
which uses state-of-the-art electroencephalography (EEG) hardware in combination with
advanced signal processing algorithms to detect sleep stages, including Wake, Light Sleep, Deep
Sleep and REM. The Zmachine was designed for use in the clinical or home environment. The
Zmachine gathers high quality, objective, epoch-by-epoch, sleep state information and summary
sleep statistics, all of which can be reviewed by a clinician either independently, or in the
presence of their patient.
The Zmachine acquires the EEG signal necessary for sleep monitoring using three high quality,
self-stick, single-use, disposable EEG sensors. The three sensors are designed for easy, self-
application by the patient with minimal skin preparation (alcohol wipe and dry). The sensors are
located completely outside of the hairline on the mastoids (signal) and the back of the neck
(ground). Integrated impedance measurement technology verifies the quality of the sensor
connections immediately prior to the start of monitoring and at periodic intervals during use.
Indications for Use
The Zmachine is a single-channel, EEG acquisition and analysis system, designed for use in the
home or clinical environments. This device is intended to be used by qualified healthcare
practitioners to monitor the wake and sleep states of adult patients and as an adjunct to their
diagnosis of sleep disorders.

2 Introduction
The Zmachine at a Glance
FRONT SIDE
SYSTEM DISPLAY
System Display
System Buttons
Sensor/Charger
Port
MicroSD
Socket
Battery Status
Operating Mode
Information Area

Introduction 3
Description of Reusable Components
The Zmachine system may include some or all of the reusable items described below.
Zmachine
Rating: 5VDC ~ 600mA
GSC P/N 200-160B (Blue)
GSC P/N 200-160G (Green)
Zmachine Sensor Cable
Silicone jacket / 1.2m length
GSC P/N 200-017
Zmachine System Charger
IN: 90-264VAC ~ 0.1A,
47-63Hz || OUT: 5VDC ~ 1.2A
GSC P/N 200-028
MicroSD Card
GSC P/N 200-051-4G
USB-MicroSD Card Reader
GSC P/N 200-070
Zmachine Clinician Key
GSC P/N 200-034
Zmachine Travel Case
GSC P/N 200-120
Zmachine Hanging Cover
GSC P/N 200-154
Note: Due to differences in brand and model numbers, part appearances may vary.
Only use General Sleep approved parts and accessories.

4 Introduction
Description of Disposable Components
The Zmachine system may include some or all of the disposable items described below.
Zmachine Patient Sensors
GSC P/N 200140-1
Alcohol Swabs
GSC P/N 200-060
Note: Due to differences in brand and model numbers, part appearances may vary.
Only use General Sleep approved parts and accessories.
This manual suits for next models
1
Table of contents
Popular Measuring Instrument manuals by other brands

Powerfix Profi
Powerfix Profi 278296 Operation and safety notes

Test Equipment Depot
Test Equipment Depot GVT-427B user manual

Fieldpiece
Fieldpiece ACH Operator's manual

FLYSURFER
FLYSURFER VIRON3 user manual

GMW
GMW TG uni 1 operating manual

Downeaster
Downeaster Wind & Weather Medallion Series instruction manual

Hanna Instruments
Hanna Instruments HI96725C instruction manual

Nokeval
Nokeval KMR260 quick guide

HOKUYO AUTOMATIC
HOKUYO AUTOMATIC UBG-05LN instruction manual

Fluke
Fluke 96000 Series Operator's manual

Test Products International
Test Products International SP565 user manual

Sensa Core
Sensa Core Lacto Spark user manual