Monitored Therapeutics, Inc. © Copyright 2020 2 P/N 45-50035 Rev. K, 2020-10-21
Contents
1. INTRODUCTION...................................................................................................................................................4
2. INDICATION FOR USE .........................................................................................................................................4
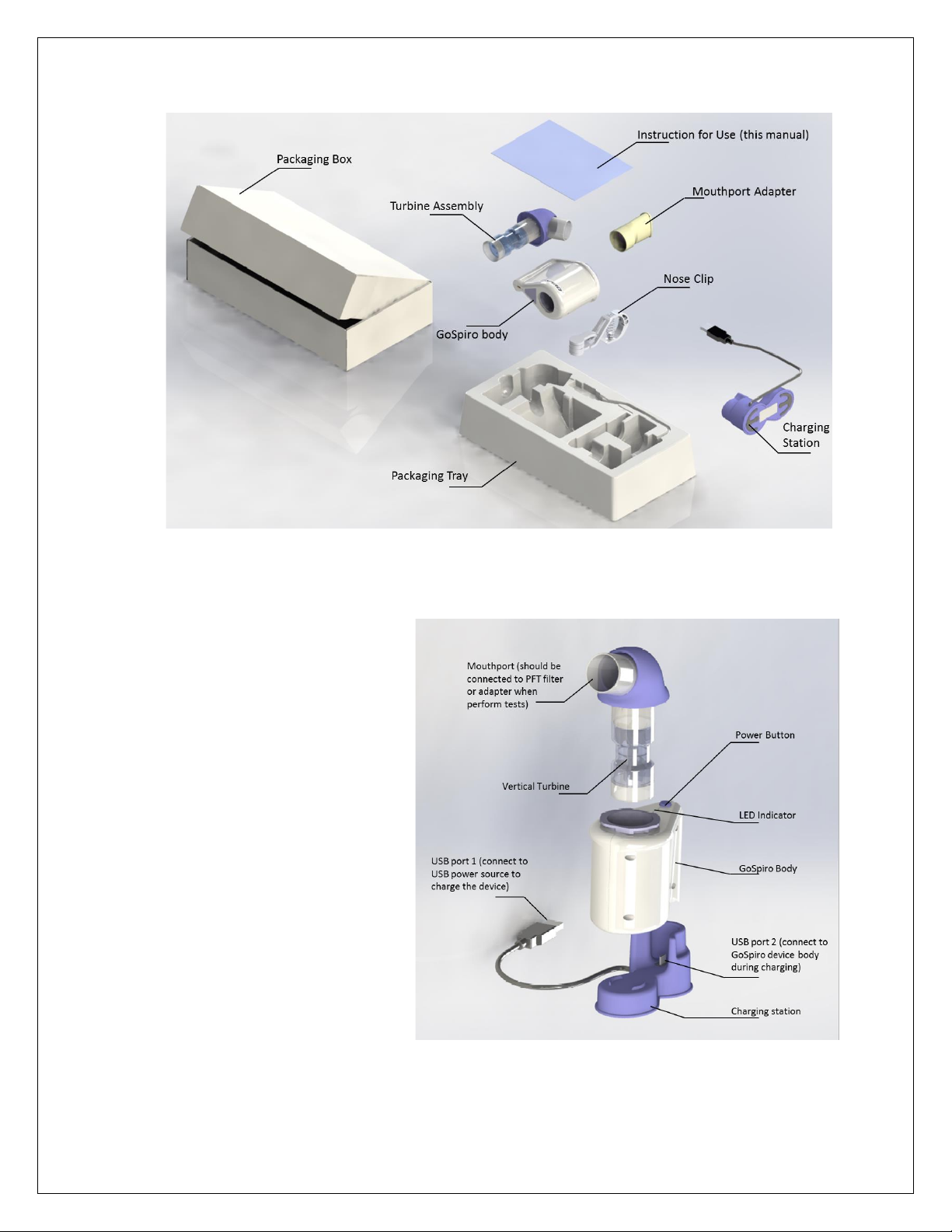
3. PACKAGE CONTENTS..........................................................................................................................................5
4. DESCRIPTION OF GOSPIRO ................................................................................................................................ 5
5. WARNINGS AND CAUTIONS ...............................................................................................................................6
6. CONTRAINDICATIONS.........................................................................................................................................8
7. ENVIRONMENT ...................................................................................................................................................8
8. GETTING STARTED .............................................................................................................................................9
9. PAIRING YOUR GOSPIRO®WITH A GOHOME™AND/OR SMARTPHONE ......................................................10
10. PERFORMING TESTS.........................................................................................................................................11
11. BATTERY MANAGEMENT .................................................................................................................................12
12. CALIBRATION....................................................................................................................................................13
13. CLEANING..........................................................................................................................................................13
14. HIGH LEVEL DISINFECTION.............................................................................................................................15
15. ACCESSORIES ....................................................................................................................................................15
16. MAINTENANCE .................................................................................................................................................16
17. SERVICING.........................................................................................................................................................16
18. WARRANTY AND LIABILITY.............................................................................................................................17
19. TROUBLE SHOOTING INFORMATION...............................................................................................................18
20. ELECTROMAGNETIC COMPATIBILITY..............................................................................................................19
21. BLUETOOTH®WIRELESS COMMUNICATION ..................................................................................................21
22. SYMBOLS ...........................................................................................................................................................21
23. SPECIFICATIONS ...............................................................................................................................................23
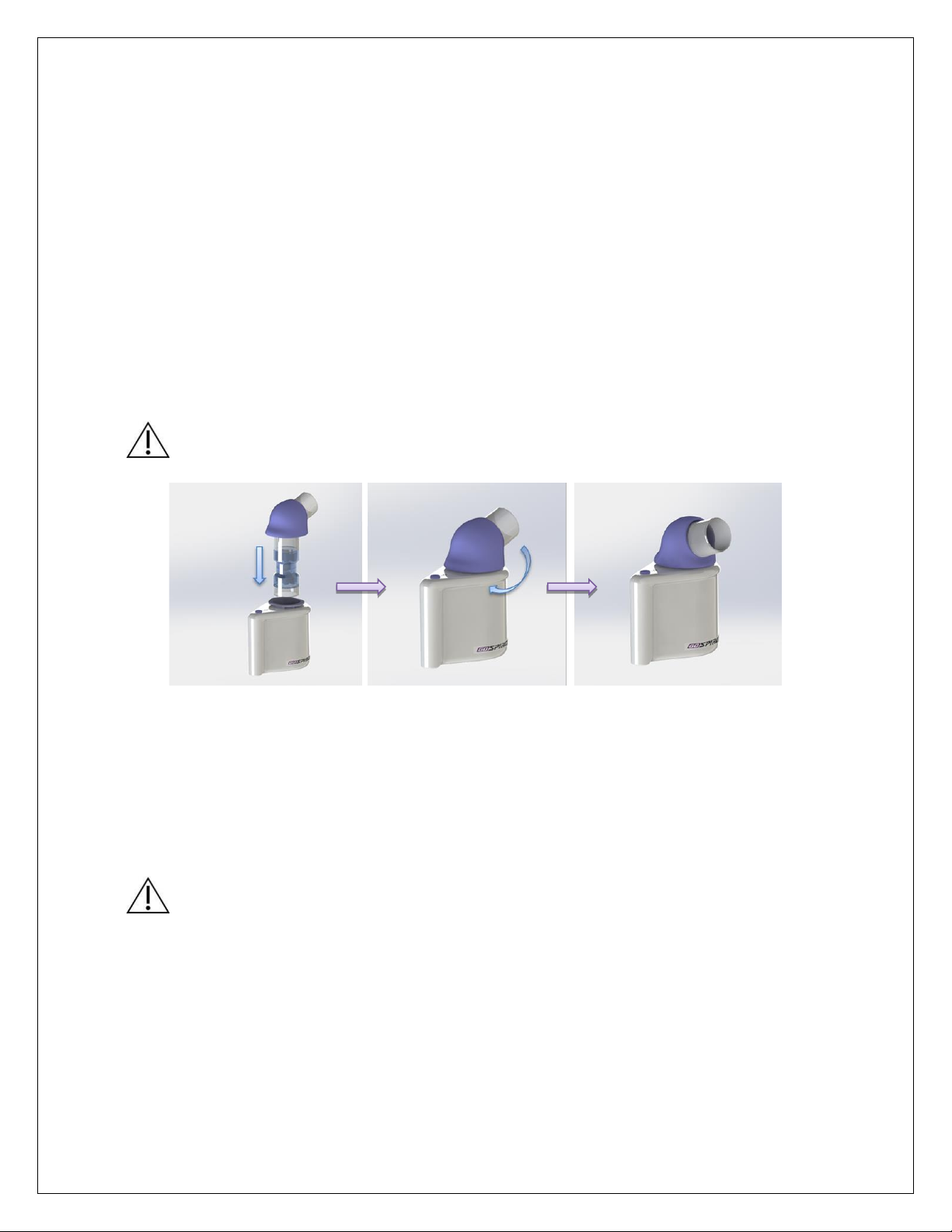
24. QUICK START ASSEMBLY GUIDE .....................................................................................................................25
25. QUICK START TESTING GUIDE ........................................................................................................................26
26. QUICK START CHARGING GUIDE .....................................................................................................................27
27.QUICK START CLEANING GUIDE .....................................................................................................................28