nobel biocare snappy User manual
Snappy™Abutment
Instructions for use
Important: Please read.
Disclaimer of liability:
This product is part of an overall concept and may only be used in conjunction with the
associated original products according to the instructions and recommendation of
Nobel Biocare. Non-recommended use of products made by third parties in conjunction
with Nobel Biocare products will void any warranty or other obligation, express or
implied, of Nobel Biocare. The user of Nobel Biocare products has the duty to determine
whether or not any product is suitable for the particular patient and circumstances.
Nobel Biocare disclaims any liability, express or implied, and shall have no responsibility
for any direct, indirect, punitive or other damages, arising out of or in connection with any
errors in professional judgment or practice in the use of Nobel Biocare products. The user
is also obliged to study the latest developments in regard to this Nobel Biocare product
and its applications regularly. In cases of doubt, the user has to contact Nobel Biocare.
Since the utilization of this product is under the control of the user, they are his/her
responsibility. Nobel Biocare does not assume any liability whatsoever for damage arising
thereof. Please note that some products detailed in this Instruction for Use may not be
regulatory cleared, released or licensed for sale in all markets.
Description:
A premanufactured dental implant abutment directly connected to the endosseous dental
implant intended for use as an aid in prosthetic rehabilitation.
Internal conical connection for NobelActive® CC, NobelReplace® CC and NobelParallel™ CC.
Internal tri-channel connection for: NobelReplace®, Replace Select™ and NobelSpeedy®
Replace.
External hex connection for: Brånemark System® and NobelSpeedy® Groovy.
Intended use:
Dental implant abutments are intended to be used in the upper or lower jaw and used for
supporting tooth replacements to restore chewing function.
The abutments in combination with two-stage endosseous implants are used as the foun-
dation for anchoring tooth replacements in either jaw. Restorations range from replacing
one single tooth to fixed partial dentures using cement- retained supra-constructions.
Indications:
Snappy™ abutment is a premanufactured prosthetic component directly connected to
the endosseous dental implant and is intended to as an aid in temporary and permanent
prosthetic rehabilitation.
Contraindications:
Snappy™ abutment is contraindicated in patients:
– who are medically unfit for an oral surgical procedure.
– in whom adequate sizes, numbers or desirable positions of implants are not reachable
to achieve safe support of functional or eventually parafunctional loads.
– allergic or hypersensitiv to commercially pure titanium, titanium alloy Ti-6Al-4V
(titanium, aluminum, vanadium), polycarbonate and polysulfone.
Cautions:
Pre-operative hard tissue or soft tissue deficits may yield a compromised esthetic result or
unfavorable implant angulations.
To secure the long term treatment outcome it is advised to provide comprehensive regu-
lar patient follow up after implant treatment and to inform about appropriate oral hygiene.
All instruments and tooling used in surgery must be maintained in good condition and
care must be taken that instrumentation does not damage implants or other components.
Special attention has to be given to patients who have local or systemic factors that could
interfere with the healing process of either bone or soft tissue or the osseointegration
process (e.g., cigarette smoking, poor oral hygiene, uncontrolled diabetes, oro-facial
radiotherapy, steroid therapy, infections in the neighboring bone).
Special caution is advised in patients who receive bisphosphonate therapy.
In general, implant placement and prosthetic design must accommodate individual
patient conditions. In case of bruxism or unfavorable jaw relationships reappraisal of the
treatment option may be considered.
Never exceed 35 Ncm prosthetic tightening torque for the abutment screw. Overtightening
of abutment may lead to a screw fracture.
Because of the small size of the devices, care must be taken that they are not swallowed
or aspirated by the patient.
It is strongly recommended that clinicians, new as well as experienced implant users,
always go through special training before undertaking a new treatment method.
NobelBiocare offers a wide range of courses for various levels of knowledge and
experience. For more info please visit www.nobelbiocare.com.
Working the first time with a colleague, experienced with the new device/treatment
method, avoids eventual complications. Nobel Biocare has a global network of mentors
available for this purpose.
Close cooperation between surgeon, restorative dentist and dental laboratory technician
is essential for a successful implant treatment.
Handling procedure:
Modifications of abutments could be performed using copious water irrigation using
high-speed drilling device and a fine diamond drill.
Note: Occlusal reduction of the Snappy™ Abutment should not be performed when plan-
ning to use Snappy™ impression coping as retention may be compromised.
Clinical procedure:
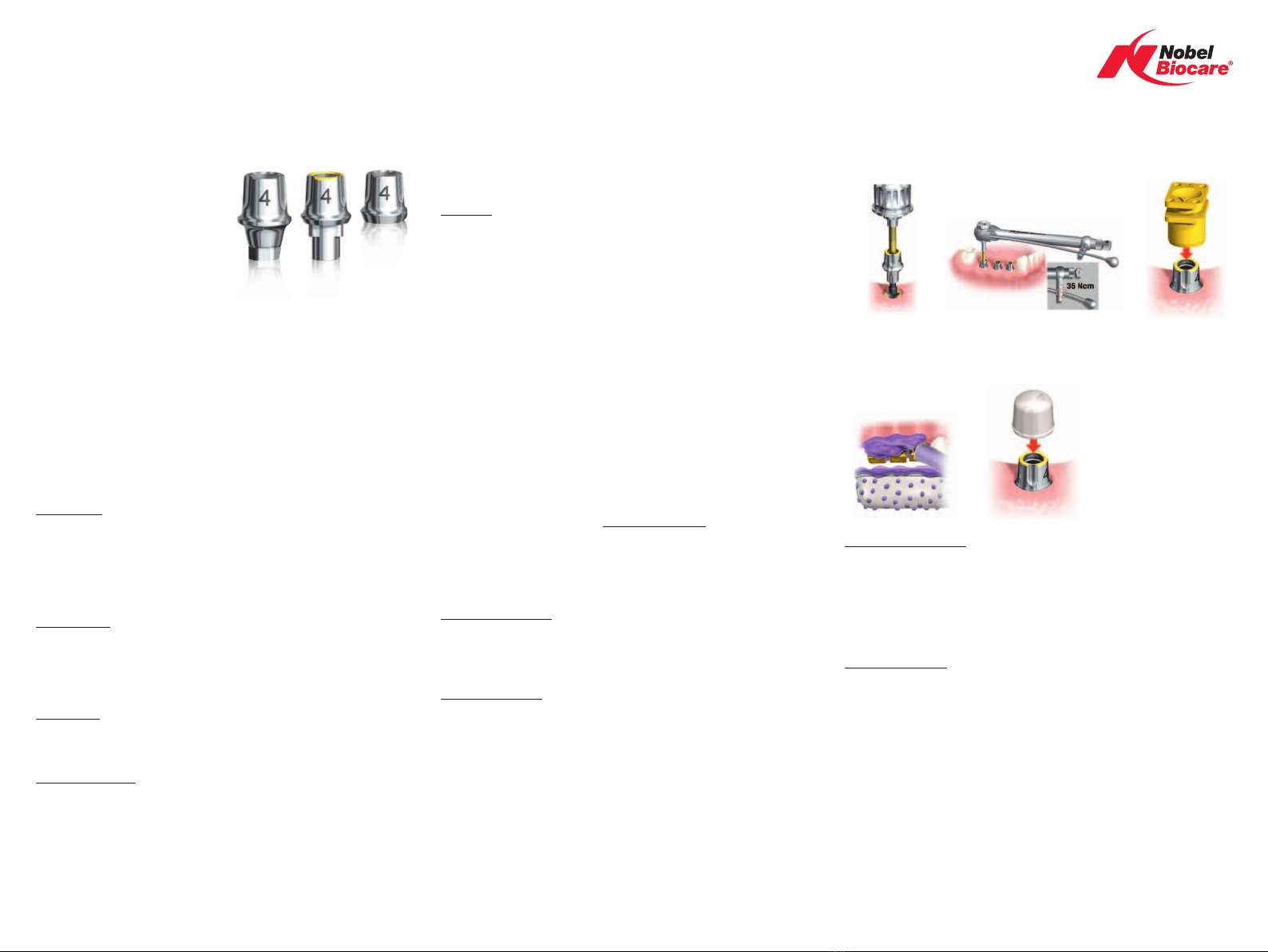
1. Select appropriate abutment and check occlusal clearence.
2. Connect the abutment (A). It is recommended to verify the final abutment seating
using radiographic imaging.
3. Tighten the abutment to 35 Ncm using Unigrip™ Screwdriver and Manual Torque
Wrench prosthetic (B).
Caution: Never exceed recommended maximum 35 Ncm prosthetic tightening torque
for the abutment screw. Over tightening of abutment may lead to a screw fracture.
4. Press the impression coping onto the abutment. A “snap” indicates that the impres-
sion coping is in place (C).
ACB
5. Take an impression (D)
6. Provisionalize using plastic temporary coping or a healing cap (E).
Caution: Do not use plastic temporary coping with polyurethane cements. The cement
will not cure.
DE
Laboratory procedure:
7. Produce a working model with removable gingival material.
Caution: When fabricating the model, use only Snappy™ Abutment 4.0 Abutment
Replica in corresponding 4.0 Impression Coping and Snappy™ Abutment 5.5 Abutment
Replica in corresponding 5.5 Impression Coping. Verify correct fit before casting the model.
8. Fabricate a crown or bridge with NobelProcera® or with conventional casting
technique using the plastic copings as burn-out patterns.
9. Complete the restoration with ceramic if applicable.
Clinical procedure:
10. Remove temporary restoration if applicable.
11. Use the Unigrip™ Screwdriver and Manual Torque Wrench prosthetic to verify the
tightening oftheabutment to 35 Ncm.
Caution: Never exceed recommended maximum 35 Ncm prosthetic tightening
torque for the abutment screw. Over tightening of abutment may lead to a screw
fracture.
12.
Cement the final restoration using conventional procedures after sealing of
access hole (F).
Caution: Do not use temporary cement when cementing ceramic crowns and
bridges, due to increased risk of microfractures.
1/2
IFU1017 000 00
F
Snappy™ Abutment screw chart
Abutment screw
(clinical)
Lab screw
Internal tri-channel connection NP 36818 31170
Internal tri-channel connection RP, WP, 6.0 29475 29293
External hex connection NP 29282 31168
External hex connection RP 29283 29290
External hex connection WP 29284 31169
Internal conical connection NP 37891 37894
Internal conical connection RP/WP 37892 37895
For additional information on restorative and dental laboratory procedures please consult
treatment guidelines available at www.nobelbiocare.com or request latest printed version
from a Nobel Biocare representative.
Materials:
Abutments with internal conical and tri-channel connection: Titaniumalloy 90% Ti, 6% Al, 4% V.
Abutments with external hex connection: commercially pure titanium.
Abutment screws: Titanium alloy 90% Ti, 6% Al, 4% V.
Temporary Coping: Polycarbonate (PC).
Healing Cap: Polysulfone (PS).
Cleaning and sterilization instructions:
Snappy™ Abutment is delivered sterile for single use only prior to the labeled expiration date.
Warning: Do not use if package is damaged or previously opened.
Caution: Snappy™ Abutment is a single use product that must not be reprocessed.
Reprocessing could cause loss of mechanical, chemical and / or biological characteristics.
Reuse could cause cross contamination.
MR safety information:
Note: Only the Conical Connection Wide Platform has been assessed as MR Conditional.
The other NobelActive® platform sizes have not been evaluated for safety and compatibility
in the MR environment and have not been tested for heating or migration in the MR
environment
Non-clinical testing has demonstrated that the device is MR conditional. A patient with
this device can be safely scanned in a MR system meeting the following conditions:
– Static magnetic field of 1.5-Tesla and 3.0-Tesla only.
– Maximum spatial gradient magnetic field of 4000 Gauss/cm (40 T/m).
– Maximum MR system reported, whole body averaged specific absorption rate (SAR) of
2 W/kg (Normal Operating Mode) or 4 W/kg (First Level Controlled Mode).
Under the scan conditions defined above, the device is expected to produce a maximum
temperature rise of 4.1°C after 15 minutes of continous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately
30 mm from the product when imaged with a gradient echo pulse sequence and a 3 Tesla
MRI system.
Removable restorations should be taken out prior to scanning, as is done for watches,
jewelry etc.
Should there be no MR symbol on the product label, please note that the product has not
been evaluated for safety and compatibility in the MR environment. The product has not
been tested for heating or migration in the MR environment.
For additional information on Magnetic Resonance Imaging, please consult the
“Cleaning & Sterilization Guidelines including MRI Information of Nobel Biocare
Products” available at www.nobelbiocare.com/sterilization or request latest printed
version from a Nobel Biocare representative.
Storage and handling:
The product must be stored in a dry place in the original packaging at room temperature
and not exposed to direct sunlight. Incorrect storage may influence device characteristics
leading to failure.
Disposal:
Disposal of the device shall follow local regulations and environmental requirements,
taking different contamination levels into account.
Manufacturer: Nobel Biocare AB, Box 5190, 402 26
Västra Hamngatan 1, 411 17 Göteborg, Sweden.
Phone: +46 31 81 88 00. Fax: +46 31 16 31 52. www.nobelbiocare.com
Canada license exemption: Please note that not all products may have been licenced in
accordance with Canadian law.
Prescription device: Rx only
Caution: Federal law restricts this device tosale by or on the order of a licensed physician
or dentist.
EN
All rights reserved.
Nobel Biocare, the Nobel Biocare logotype and all other trademarks used in this document
are,
if nothing else is stated or is evident from the context in a certain case, trademarks
ofNobel Biocare. Product images in this folder are not necessarily to scale.
2/2
Do not use if package
is damaged
Use-by date Do not re-use
Consult instructions
for use
Do not
resterilize
Batch code
Magnetic resonance
conditional
Sterile using
irradiation
IFU1017 000 00
Other nobel biocare Dental Equipment manuals


















