DUKE S.T.01/3AR rev.0 4/2011
1 GENERAL INFORMATION
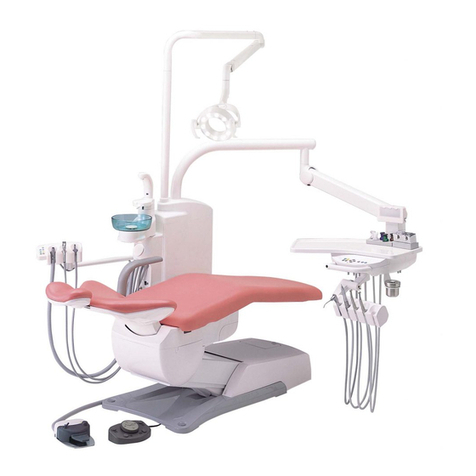
Duke offers a wide range of instruments to be positioned in the four existing housings.
The instrument table is made entirely of porcelain and has no interstices, allowing for easier hygiene.
The arm is fixed to the base of the chair, allowing the table to rotate 270° around the patient for use by right
and left handed operators in a single appliance.
High speed aspiration tubes and connections are easy to remove for disinfection purposes. The filter too can be
extracted in an easy and hygienic manner.
The dental chair was designed to guarantee maximum safety and comfort. The chair back is ultra flat and has no
protruding parts at its bottom, thus enabling the operating staff to be positioned correctly when the patient is
sitting on it. At the same time, it has an anatomic shape that enables the load of the patient’s body to be
distributed in the best and most uniform way possible regardless of his/her size. The chair back can also perform
a compensation movement that prevents irritating pulling of patient’s clothes during the descent phase and
headrest adjustment after this movement.
The backrest is fitted with a safety system which locks all downwards movements in the event of interference
with foreign bodies (e.g. the operator's legs).
1.1 SAFETY RULES
-Warning: To prevent the risk of electrical shocks, the equipment must be connected exclusively to power lines
provided with a grounding system in compliance with the laws in force in the country of use.
-Before powering the equipment after installation, a repair or technical service, check and, if necessary, hook
up the connection of the grounding cables to the screw identified by the grounding system symbol.
-This equipment must be installed in rooms where the existing electrical systems conform to the regulations in
place in the installation country.
-The equipment must be installed by an authorised OMS technician; the choice of piping is made by the
designer of the system and this must be laid by a technician qualified pursuant to the laws in force in the
country of installation.
-Non-professional operators or operators who have not read this instruction manual must not be allowed to
operate the equipment.
-Always make sure that the equipment is in good operating condition.
-Do not operate the equipment if one of its parts is defective or worn. If this is the case, ask for the help of
authorised O.M.S. technicians.
-Replace defective or worn parts using genuine spare parts only that are guaranteed by O.M.S..
-Do not operate the equipment on patients with pacemakers.
-The equipment is not suitable for operation in the presence of a flammable anaesthetic mix of air and oxygen
or nitrous oxide.
-Do not operate the equipment in the event of liquid spillage on the floor.
-Tips and dental drills for micromotors and turbines are not included in O.M.S. supply. We recommend using
parts conforming to the standard ISO 10993-1 that have to be cleaned and sterilised according to the
methods defined by their manufacturers.
-Warning: do not modify this equipment without prior authorisation by the manufacturer.
-Maintenance operations must be performed after switching the equipment off and without a patient sitting in
it.
-Warning: some parts, identified by the symbol illustrated in figure 1 “WARNING – LIVE PARTS”, are
energised with mains voltage even after switching off the power switch. If these parts require service, cut
out voltage to the system supplying power to the equipment, before making any operations.
-Warning: the power switch isolates the equipment from the mains electricity. So, before performing any
operations in the equipment, make sure that the power switch is off.
-To ensure prevention of dental chair movements during specific operations requiring this option, it is
necessary to enable the relevant function (see section 4.3.6 DENTAL CHAIR MOVEMENT STOP).
-Connection to a suction unit must be made in compliance with the instructions given in this manual and in the
wiring diagram; the suction unit must be CE marked pursuant to EC Directive 93/42/EEC and amendments,
“Medical Equipment” and with international safety standards IEC EN 60601-1 (Medical electrical equipment –
General requirements for Safety) and IEC EN 60601-1-2 (Medical electrical equipment –Collateral standard:
Electromagnetic Compatibility).