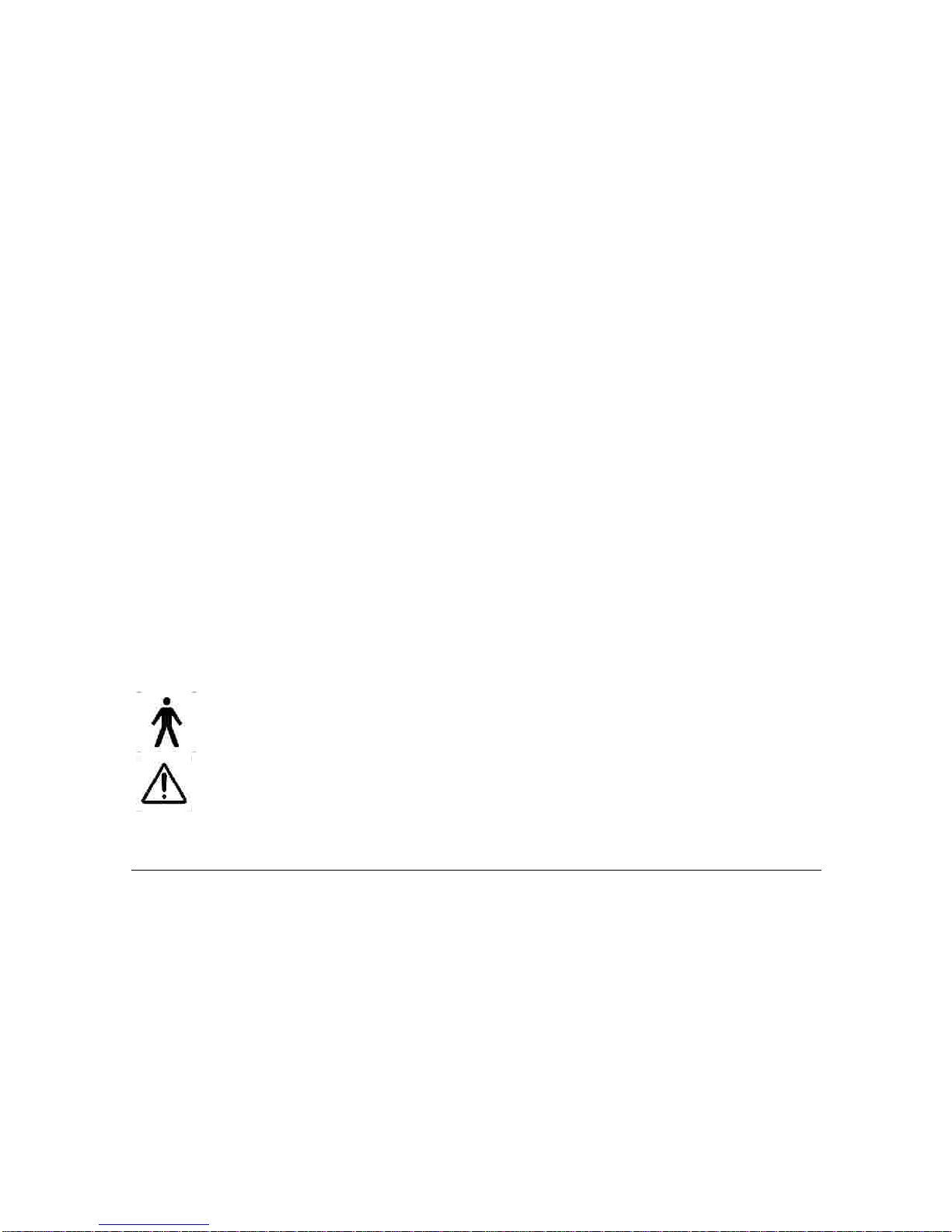
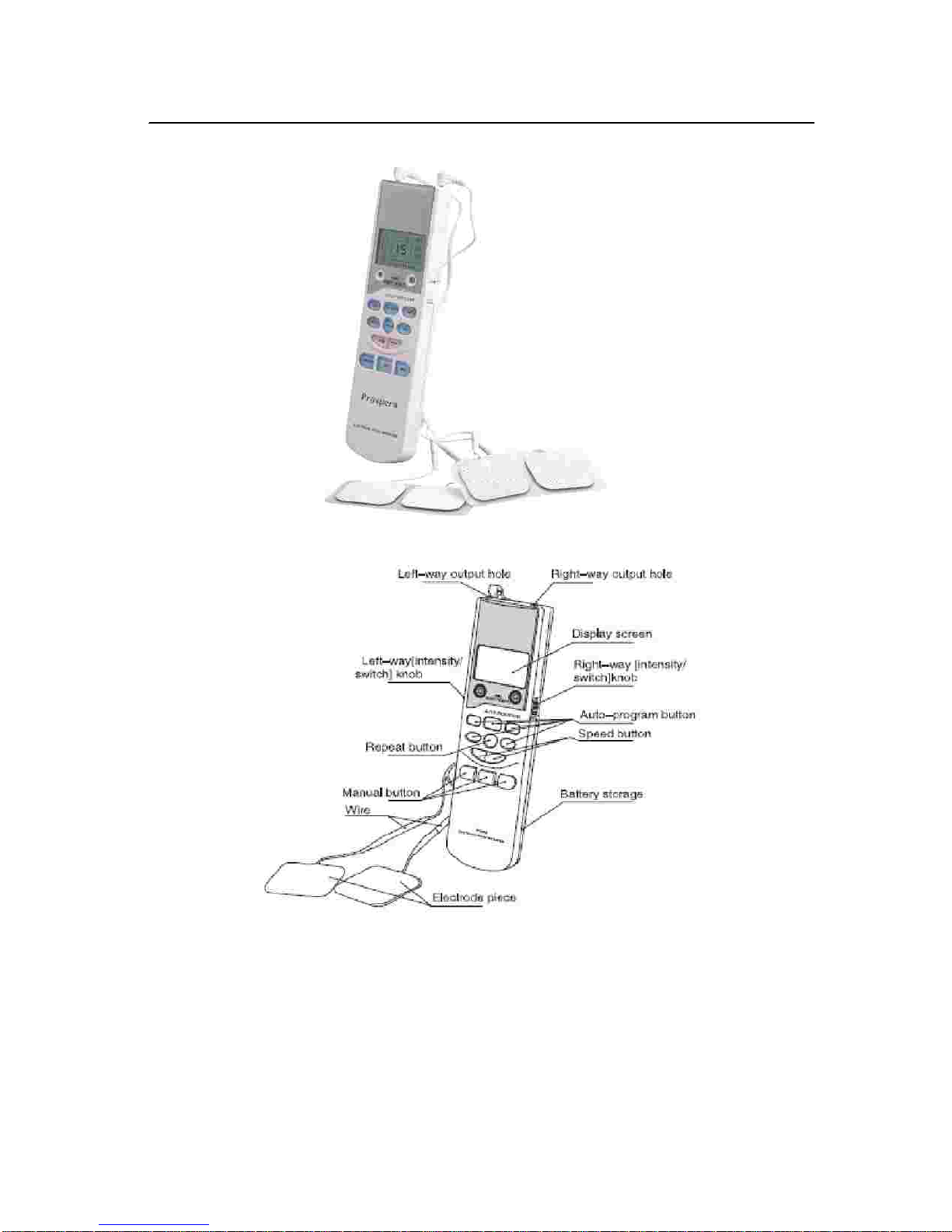
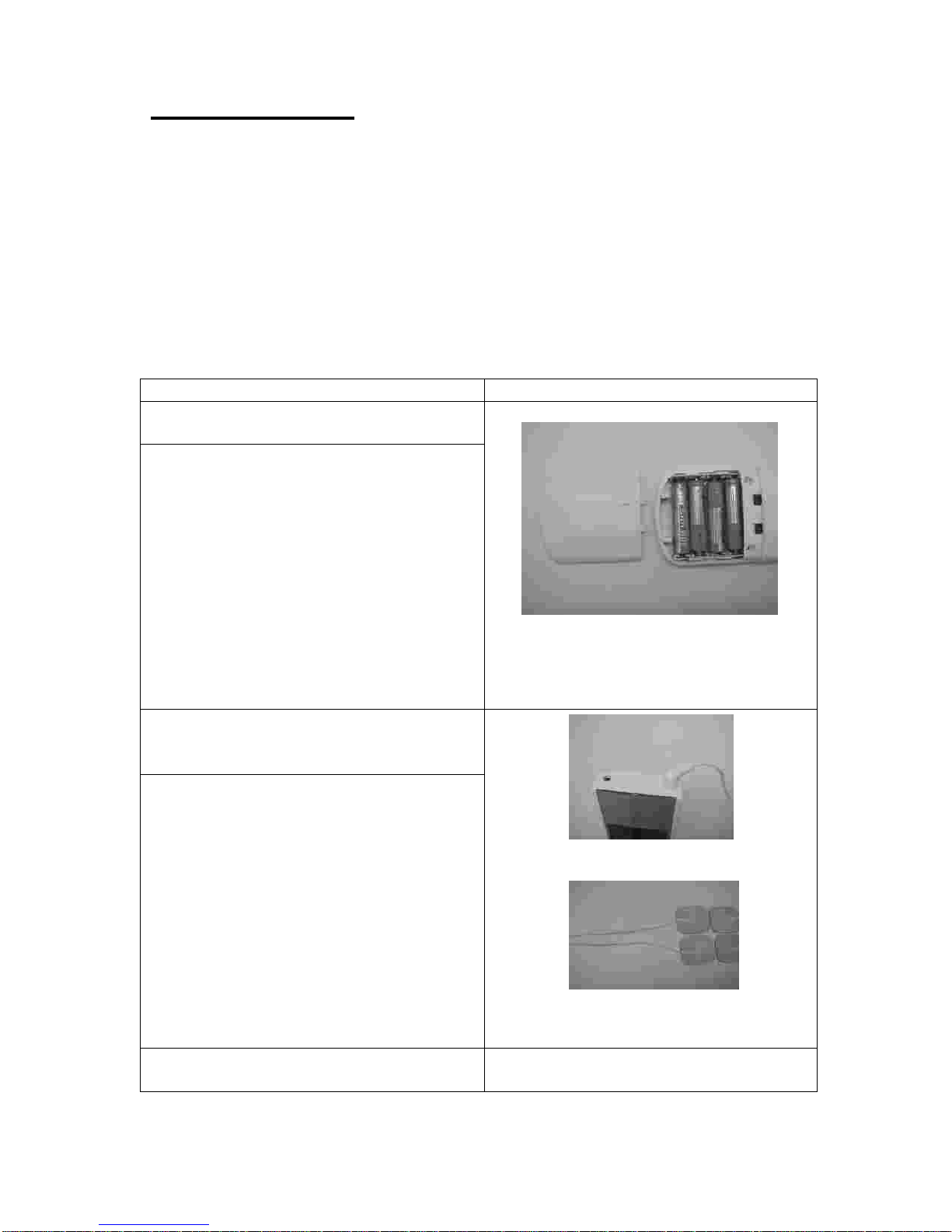
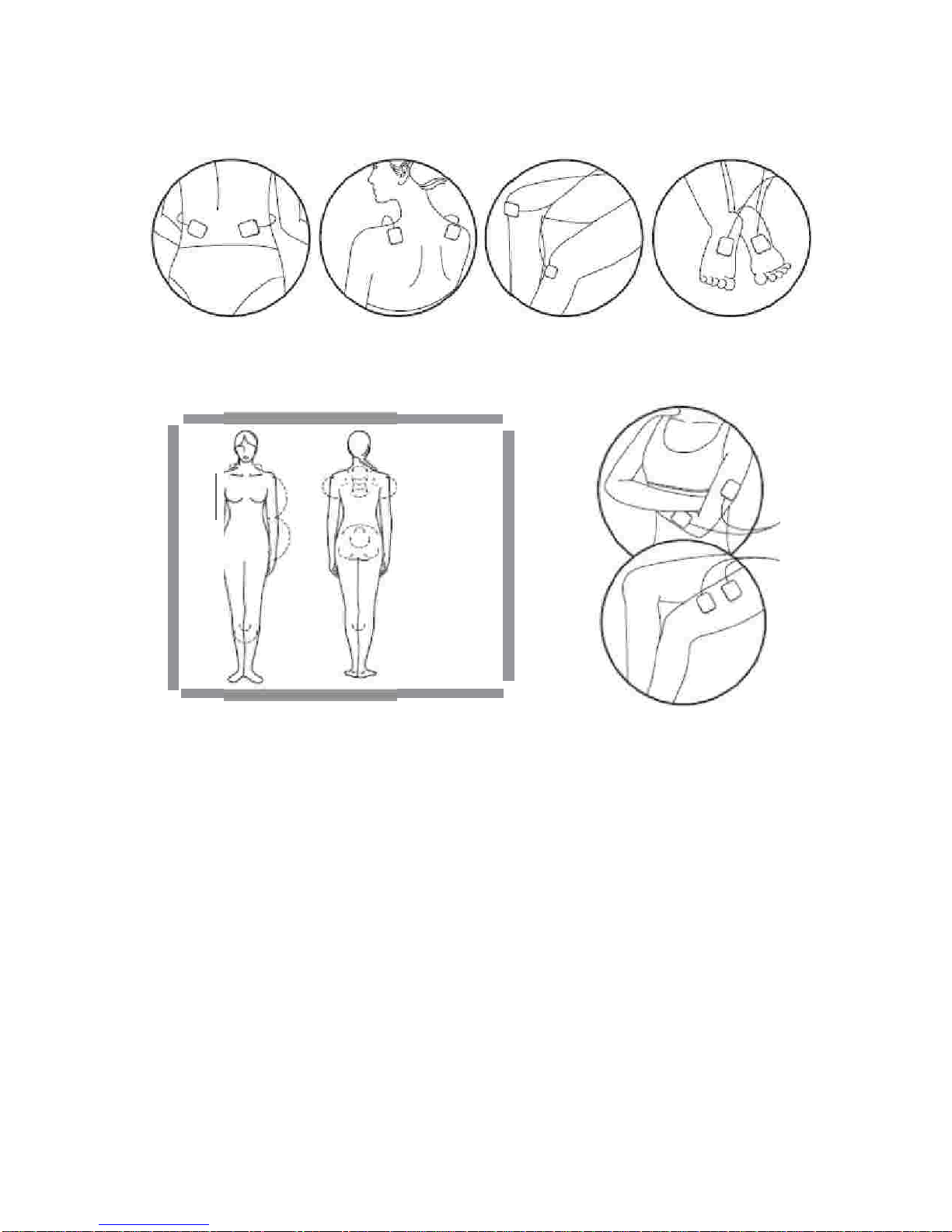
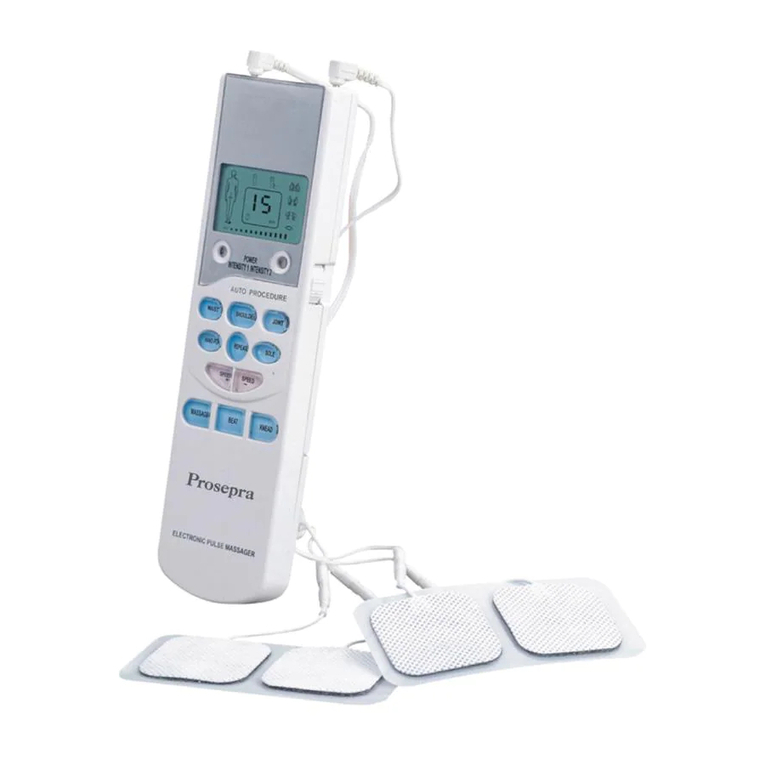
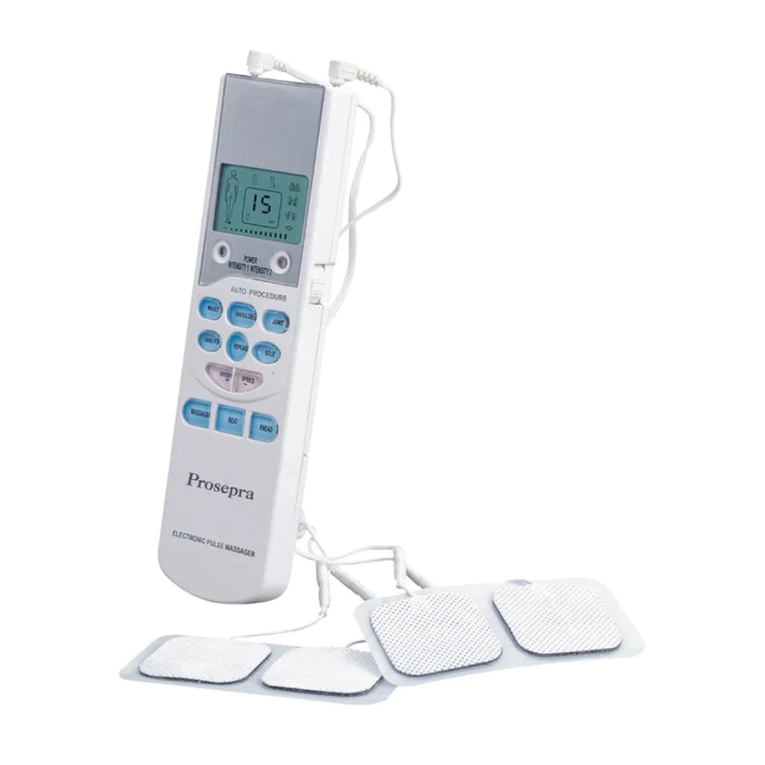
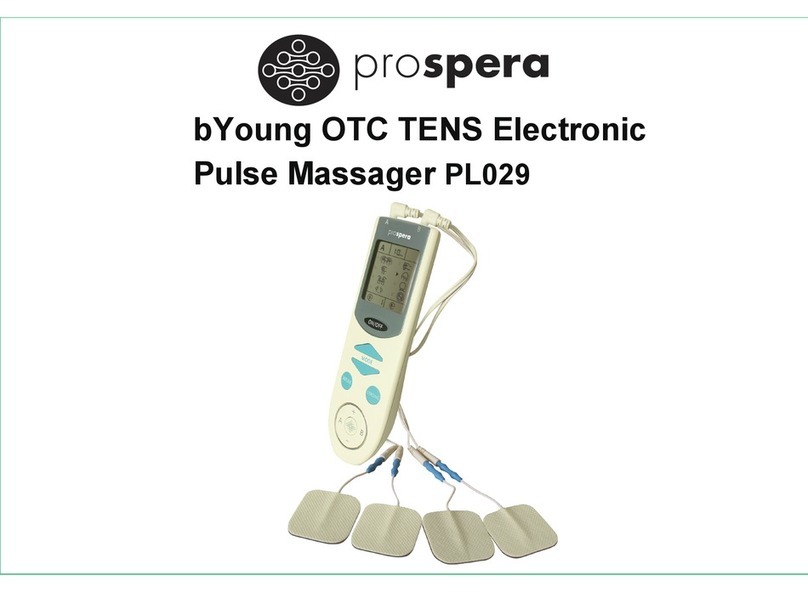
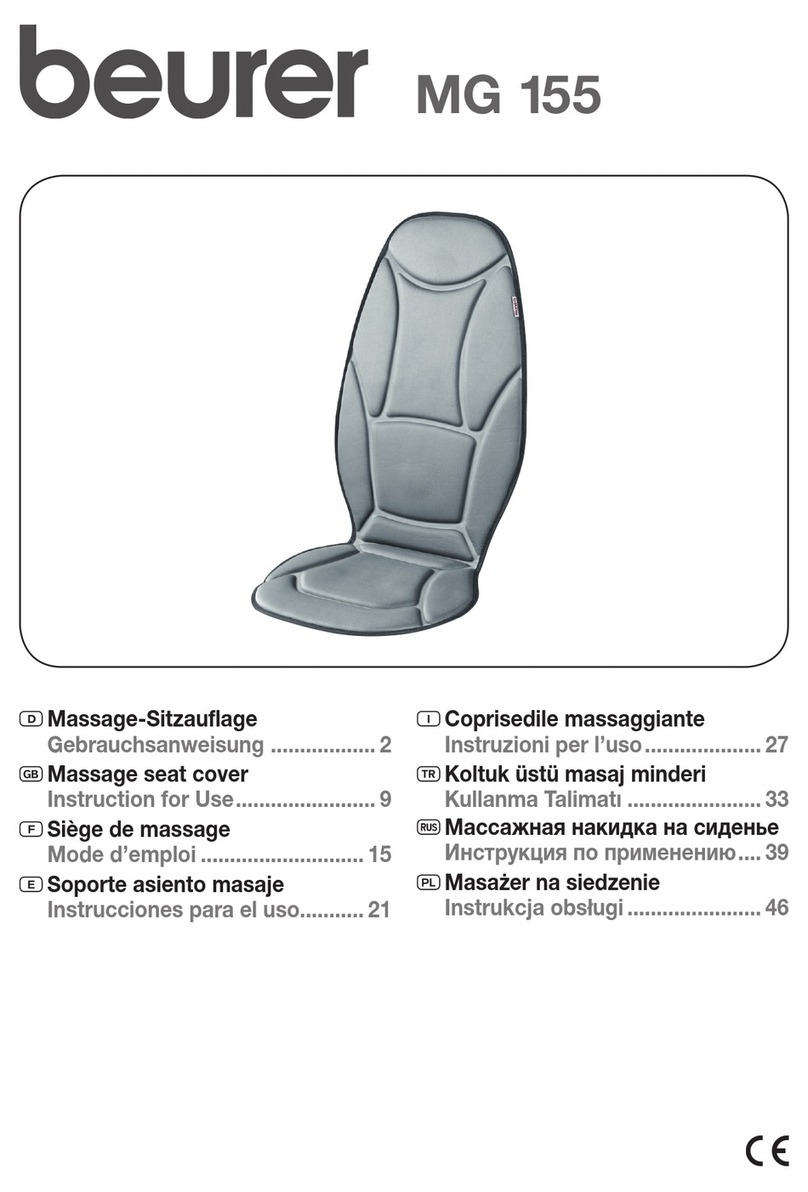
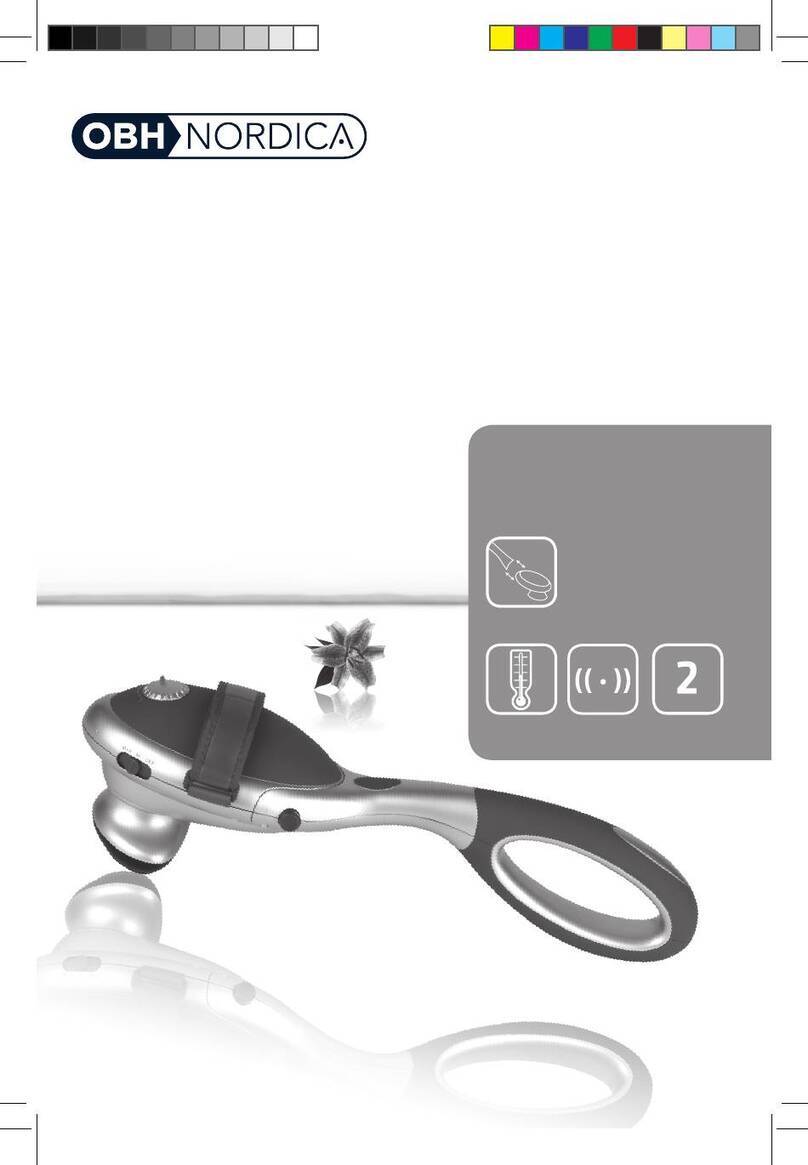
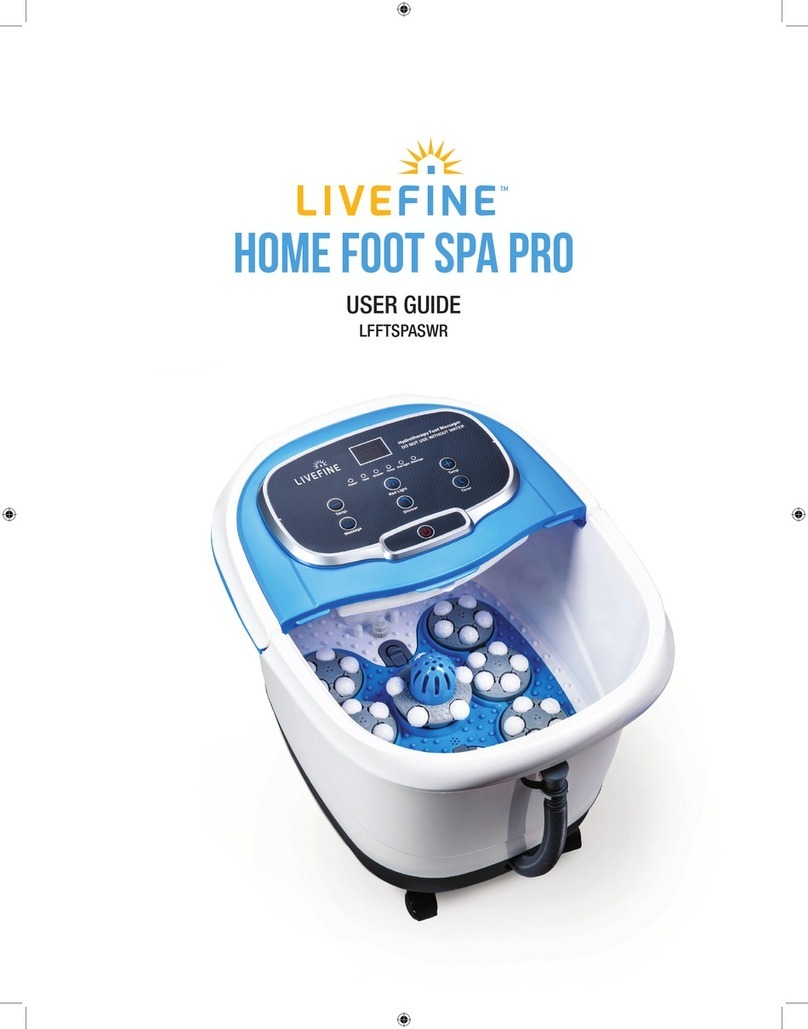
Do not use this device if you have a cardiac pacemaker, implanted defibrillator, or other implanted
metallic or electronic device. Such use could cause
electric shock, burns, electrical interference, or
If you are in the care of a physician, consult with your physician before using this device;
on your heart, head, mouth, pudendum or blemished skin areas;
ulation across your chest because the introduction of electrical current into the
chest may cause rhythm disturbances to your heart, which could be lethal;
Do not apply during pregnancy.
Do not apply stimulation over painful areas.
If you have painful areas, you should consult with your
physician before using this device;
Do not apply stimulation over open wounds or rashes, or over swollen, red, infected, or inflamed
ons (e.g., phlebitis, thrombophlebitis, varicose veins);
Do not apply stimulation over, or in proximity to, cancerous lesions;
Do not apply stimulation in the presence of electronic monitoring equipment (e.g., cardiac monitors,
ECG alarms), which may not
operate properly when the electrical stimulation device is in use;
Do not apply stimulation when in the bath or shower;
Do not apply stimulation while sleeping;
Do not apply stimulation while driving, operating machinery, or during any activity in
electrical stimulation can put you at risk of injury;
Do not use the device on children;
Consult with your physician before using this device, because the device may cause lethal rhythm
disturbances to the heart in susceptible individuals; and
stimulation only to normal, intact, clean, healthy skin;
Do not use this device in high humidity areas such as a bathroom;
Do not attempt to move the electrode heads while the device is operating; and
Keep the device away from wet, high temperature and di
. Shady and dry place is good
This device is not effective for pain of central origin, including headache;
This device is not a substitute for pain medications and other pain management therapies;
This device is a symptomatic treatment and, as such, suppresses the sensation of pain that would
otherwise serve as a protective mechanism;
term effects of electrical stimulation are unknown;
Since the effects of stimulation of the
brain are unknown, stimulation should not be applied across
your head, and electrodes should not be placed on opposite sides of your head;