Stim Wave Technologies PDBT-915-2K User manual

WEARABLE ANTENNA ASSEMBLY
USER MANUAL
Caution: Federal law restricts this device to sale by or on the order of a physician.
WEARABLE ANTENNA ASSEMBLY KIT (PDBT-915-2K)
WEARABLE ANTENNA ASSEMBLY
(PDBT-915-2A)
English
Page | 2
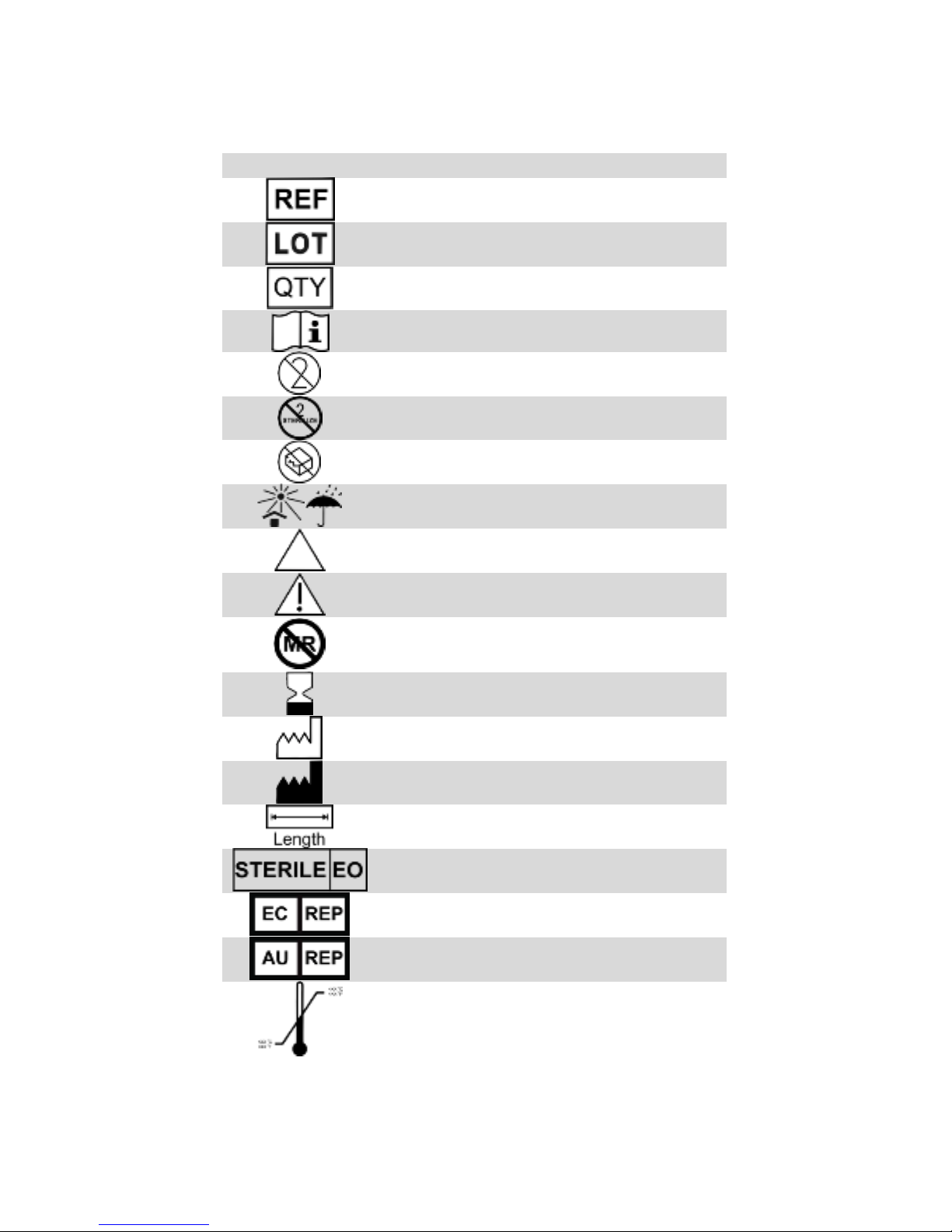
EXPLANATION OF SYMBOLS ON PRODUCT OR PACKAGE
Refer to the appropriate product for symbols that apply.
Symbol
English –EN
Device reference identification
Lot number
Quantity of product included in package
Consult instructions for use
Do not reuse
Do not resterilize
Do not use if package is damaged
Store in a cool, dark, dry place
Caution
Warning
MR Unsafe
Use by
Manufacturing date
Manufacturer
Device length
Sterilization: ethylene-oxide gas
European Authorized Representative
Australian Sponsor
Temperature limits

English
Page | 3
TABLE OF CONTENTS
EXPLANATION OF SYMBOLS ON PRODUCT OR PACKAGE.....................2
HOW TO USE THIS MANUAL...............................................................4
INDICATIONS FOR USE ................................................................................ 4
SAFETY INFORMATION.......................................................................5
CONTRAINDICATIONS.................................................................................. 5
WARNINGS ............................................................................................... 6
PRECAUTIONS ......................................................................................... 11
ADVERSE EVENT SUMMARY....................................................................... 14
PARTS OF YOUR STIMULATION SYSTEM............................................15
OVERVIEW OF THE USER CONTROLS............................................................ 16
POSITIONING THE WAA............................................................................ 17
STARTING STIMULATION ........................................................................... 18
SELECTING A STIMULATION PROGRAM ........................................................ 18
INCREASING OR DECREASING AMPLITUDE .................................................... 18
MAINTENANCE ................................................................................19
BATTERY CHARGING................................................................................. 19
WHEN TO CALL YOUR CLINICIAN................................................................. 21
CLEANING AND CARE PRECAUTIONS ............................................................ 21
SAFETY AND TECHNICAL CHECKS ................................................................. 21
WAA DISPOSAL....................................................................................... 21
PATIENT IDENTIFICATION CARD .................................................................. 22
SPECIFICATIONS...............................................................................22
WIRELESS INFORMATION .................................................................23
TROUBLESHOOTING.........................................................................24
CONTACT INFORMATION .................................................................26

English
Page | 4
HOW TO USE THIS MANUAL
This manual will help you understand how to use and care for your
neurostimulator system. It also provides you with warnings and precautions
you should know about. You should discuss with your clinician any questions or
concerns you have after reading this manual. Please refer to the Freedom
Spinal Cord Stimulator (SCS) System Product Safety Guide for EMC related
safety information.
Please refer to the Freedom Spinal Cord Stimulator (SCS) System Product Safety
Sheet for EMC related safety information per IEC 60601-1-2:2007. The Product
Safety Sheet is used in conjunction with this manual as the instructions for use.
This device complies with Part 18 of the FCC Rules. Per FCC 18.213, See
Warnings section in this document for the interference potential of the device
and methods for correcting interference. See Maintenance section in this
document for the instructions on system maintenance.
Per FCC 15.21, changes or modifications not expressly approved by the party
responsible for compliance could void the user's authority to operate the
equipment.
Per FCC 15.19(a)(3) and (a)(4) This device complies with part 15 of the FCC
Rules. Operation is subject to the following two conditions: (1) This device may
not cause harmful interference, and (2) this device must accept any
interference received, including interference that may cause undesired
operation. FCC ID: 2AHXAPDBT2
INDICATIONS FOR USE
The Freedom Spinal Cord Stimulator (SCS) System is intended as the sole
mitigating agent, or as an adjunct to other modes of therapy used in a
multidisciplinary approach for chronic, intractable pain of the trunk and/or
lower limbs, including unilateral or bilateral pain. The Freedom-8A Trial Lead Kit
is only used in conjunction with the Freedom-8A Stimulator Receiver Kit, and
the Freedom-4A Trial Lead Kit is used for either the Receiver Kit Freedom-4A
Stimulator or the Receiver Kit Freedom-8A Stimulator. The trial devices are
solely used for trial stimulation (no longer than 30 days) to determine efficacy
before recommendation for a permanent (long term) device.

English
Page | 5
SAFETY INFORMATION
CONTRAINDICATIONS
•Poor surgical risks –Spinal cord stimulators should not be used on patients
who are poor surgical risks or patients with multiple illnesses or active
general infections. This includes patients who need anticoagulation
therapy that cannot be temporarily halted to accommodate the
implantation procedure.
•Pregnancy –Safety and effectiveness of the Freedom SCS System for use
during pregnancy and nursing have not been established.
•Inability to operate System –Spinal cord stimulators should not be used
on patients who are unable to understand or operate the System.
•Exposure to shortwave, microwave, or ultrasound diathermy –Diathermy
should not be operated within the vicinity of a patient implanted with a
Freedom Stimulator or when wearing the Wearable Antenna Assembly
(WAA). The energy from diathermy can be transferred through the
stimulator or WAA and cause tissue damage, resulting in severe injury.
•Implanted cardiac systems –Patients who have implanted cardiac systems
should not use the Freedom SCS System. Electrical pulses from the
Freedom SCS System may interact with the sensing operation of an
implanted cardiac system, causing the cardiac system to respond
inappropriately.
•Occupational exposure to high levels of non-ionizing radiation that may
interfere with therapy –Users who regularly work in environments with
elevated levels of non-ionizing radiation should not be implanted with the
Freedom SCS System. The energy in high-level areas can be transferred
through the stimulator and cause tissue damage, resulting in severe injury.
Examples of environments having high level non-ionizing radiation includes
radio or cell phone transmission stations, facilities using radiofrequency
heat sealers or induction heaters, electric power infrastructure controlled
environments (i.e. step down transformers or high voltage power lines).

English
Page | 6
WARNINGS
Electromagnetic interference (EMI) –EMI is a field of energy generated by
equipment found in the home, work, medical or public environments. EMI that
is very strong can interfere with System. The device includes features that
provide protection from EMI. Most electrical device and magnets encountered
in a normal day will not affect the operation of the System. However, strong
sources of EMI could result in the following:
•Serious patient injury resulting from heating of the implanted device
and damage to surrounding tissue.
•System damage, resulting in a loss of, or change in, symptom control
and requiring additional surgery.
•Operational changes to the WAA. This may cause either external
device to turn on, turn off, or to reset to factory settings. If this occurs,
the WAA need to be reprogrammed.
•Unexpected changes in stimulation, causing a momentary increase in
stimulation or intermittent stimulation. Some patients have described
as a jolting or shocking sensation. Although the unexpected change in
stimulation could feel uncomfortable, it does not damage the device
or cause a patient direct injury. In rare cases, as a result of the
unexpected changes in stimulation, patients have fallen down and
been injured.
If you suspect that your Freedom SCS System is being affected by EMI then you
should:
•Immediately move away from the equipment or object.
•Remove the WAA from the vicinity.
Machinery or heavy equipment –Machinery and heavy equipment (including
vehicles) should not be operated while using the Freedom SCS System.
Malfunction of the System could result in loss of body control, body function,
or a feeling that could render the patient incapable of controlling the system.
Stimulator fracture –If the Stimulator insulation is ruptured or pierced due to
extensive forces, unexpected changes in stimulation could result.
Electromagnetic equipment/environments –Avoidance of high
electromagnetic equipment radiators or environments is highly encouraged.
Examples of equipment and/or environments include the following:
•High-power amateur transmitters/antennas or citizen band (CB) radio
or Ham radio used for private recreation, communication, and wireless
experimentation.

English
Page | 7
•Electric arc welding or resistance welding equipment used for melting
and joining metals or plastics.
•Industrial electric induction furnace/heater or electric arc
furnace/heater used for melting metals and plastics.
•High-voltage areas identified by fenced areas, restricted access signs,
and caution signs (safe if outside the fenced area).
•Microwave transmitters identified by fenced areas, restricted access
signs, and caution signs (safe if outside the fenced area).
•Television and radio towers identified by fenced areas, restricted
access signs, and caution signs (safe if outside the fenced area).
•Linear power amplifiers used for increasing the power output of radio
transmitters, wireless communication applications, audio equipment
or other electronic equipment.
•Radio telemetry equipment used for tracking location of vehicles,
equipment or animals.
Active Implantable or Body Worn Medical Devices –Safety has not been
established for patients who use the Freedom SCS System with other active
implantable or body worn medical devices. These devices include other
neurostimulation systems, insulin pumps, automated external defibrillators
(AED), cochlear implants, and wearable medical sensors. Malfunction and/or
damage could occur to either system that could result in harm to the patient or
other people nearby.
Magnetic Resonance Imaging (MRI) –The Freedom-8A/4A SCS System is MR
Unsafe. Since the Freedom-8A/4A SCS System is MR Unsafe, the strong
magnetic field of the MR system could attract or otherwise damage the
System, and in the process cause serious harm to the patient or other people
or damage to the MR system.
The WAA component is MR Unsafe; ensure that the WAA does not enter the
MR system room. Since the WAA is MR Unsafe, the strong magnetic field of the
MR system could attract or otherwise damage the WAA, and in the process
cause serious harm to the patient or other people or damage the MR system.
Radiofrequency (RF) ablation –Safety has not been established for
radiofrequency (RF) ablation in patients with a Stimulator. RF ablation may
cause induced electrical currents that result in heating and tissue damage. Do
not use RF ablation anywhere near the Stimulator. If RF ablation is used, ensure
that ablation is not performed over or near the Stimulator.

English
Page | 8
Electrocautery –If electrocautery tools are used near the Stimulator then the
insulation can be damaged. The Stimulator may fail or conduct induced
currents. Induced electrical currents can cause heating that results in tissue
damage.
When electrocautery is necessary, these precautions must be followed:
•The WAA should be taken off.
•Bipolar cautery should be used.
•If unipolar cautery is necessary:
oOnly low-voltage modes should be used.
oThe lowest possible power setting should be used.
oThe current path (ground plate) should be kept as far away as
possible from the Stimulator.
oFull-length operating room table ground pads should not be used.
•After electrocautery, confirm that the Stimulator is working as
intended.
High-output ultrasonics / lithotripsy –Safety has not been established for
high-output ultrasonics or lithotripsy when implanted with the Freedom SCS
System. Use of lithotripsy may result in damage to the device or harm to the
patient.
Psychotherapeutic procedures –Safety has not been established for
psychotherapeutic procedures using equipment that generates
electromagnetic interference (e.g., electroconvulsive therapy, transcranial
magnetic stimulation) in patients who have spinal cord stimulators. Induced
electrical currents can cause heating that may result in tissue damage.
Radiofrequency Identification (RFID) Emitters - Theft detectors, electronic
article surveillance (EAS) systems, and radiofrequency identification systems
–Tests have been performed with an array of simulated RFID emitter systems,
and have demonstrated that the Freedom SCS System (implanted device and
WAA) can be affected by separation distances between the Freedom SCS
System and the RFID emitter of less than 3m (~10 ft.). More powerful RFID
Emitters might cause effect at farther distances. RFID emitters can be hidden or
portable and not obvious to the Stimwave user. Any RFID emitter may
temporarily interrupt stimulation, or cause elevated levels of stimulation. It is
recommended that if a patient feels a change in stimulation near a potential
RFID emitter, they promptly move away from the area and remove the WAA
from the body.

English
Page | 9
When possible, it is best to avoid RFID emitters or remove the WAA while
passing near RFID emitters. Patients with an implanted device should inform
the attendant who may be able to assist them in bypassing any RFID emitter. If
unavoidable, the patient should walk through the RFID emitter and promptly
move away from the area. Patients should not lean on scanners or linger in the
area of RFID emitters.
Computed Tomography (CT) Scanning –Safety has not been established for CT
scanning of patients with a Stimulator. X-rays from the scan could cause
unintended shocks or malfunctions of the Stimulator.
The CT operator should use CT scout views to determine if implanted medical
devices are present and their location relative to the programmed scan range.
For CT procedures in which the device is in or immediately adjacent to the
programmed scan range, the operator should:
•Remove the WAA from the CT scan range.
•Minimize X-ray exposure to the implanted device by:
oUsing the lowest possible X-ray tube current consistent with
obtaining the required image quality.
oMaking sure that the X-ray beam does not dwell over the
Stimulator for more than a few seconds.
After CT scanning directly over the implanted device:
•Place the WAA on body and turn on stimulation.
•Check for proper stimulation, and that indicator lights are operating as
expected.
•Shut off the WAA if it is suspected that the device is not functioning
properly.
Bone growth stimulators –Safety has not been established for bone growth
stimulators within the vicinity of the Freedom SCS System. Use of a bone
growth stimulator may result in damage to the device or harm to the patient.
Dental drills and ultrasonic probes –Safety has not been established for dental
drills or ultrasonic probes within the vicinity of the Freedom SCS System. Use of
dental drills or ultrasonic probes may result in damage to the device or harm to
the patient.
Electrolysis –Safety has not been established for electrolysis within the vicinity
of the Freedom SCS System. Use of electrolysis may result in damage to the
device or harm to the patient.

English
Page | 10
Laser procedures –Safety has not been established for lasers within the vicinity
of the Freedom SCS System. Use of lasers may result in damage to the device or
harm to the patient.
Radiation therapy –Safety has not been established for high radiation sources
such as cobalt 60 or gamma radiation when implanted with the Freedom SCS
System. Use of radiation therapy could cause damage to the device or harm to
the patient.
Transcutaneous electrical nerve stimulation –Safety has not been established
for use of transcutaneous electrical nerve stimulation (TENS) when implanted
with the Freedom SCS System. Use of TENS could cause the device to turn off
or intermittent/increased stimulation.
Other medical procedures –EMI from the following medical procedures is
unlikely to affect the device:
•Diagnostic ultrasound (e.g., carotid scan, Doppler studies)
•Diagnostic x-rays or fluoroscopy
•Magnetoencephalography (MEG)
•Positron emission tomography (PET) scans
•Therapeutic magnets (e.g., magnetic mattresses, blankets, wrist
wraps, elbow wraps) –Keep the magnet away from the stimulator
site. Magnetic fields will generally not affect the Stimulator.
WAA Skin Contact –Do not place the WAA directly on the skin. Direct skin
contact may cause irritation and/or sensitivity to the materials. The WAA must
be placed overtop a thin layer of clothing at all times.
Painful Stimulation –If the patient experiences painful stimulation, the
amplitude on the WAA should be decreased immediately and/or removed from
the patient’s body. Contact your clinician if this continues to occur.
Aircraft Usage –Safety has not been established for use of the Freedom SCS
System on aircrafts. Use of the Freedom SCS System on a commercial aircraft
may result in damage to the device or harm to the patient.

English
Page | 11
PRECAUTIONS
Physician training –Prescribing clinicians should be experienced in the
diagnosis and treatment of chronic intractable pain and should be familiar with
using the Freedom SCS System. Implanting clinicians should be experienced in
spinal procedures and should review the procedures described in the
Instructions for Use.
Keep the WAA dry –The WAA is not waterproof. Keep it dry to avoid damage.
Do not use the WAA when engaging in water activities.
Clean the WAA –Clean the outside of the WAA with a damp cloth when
needed to prevent dust and grime. Mild household cleaners will not damage
the device or labels.
Handle the WAA with care –The WAA is a sensitive electronic device. Avoid
dropping the device onto hard surfaces. Keep the WAA out of the reach of
children and pets.
Storage temperatures –The Freedom SCS System should be kept within the
storage temperatures listed on product packaging. Exceeding the storage
temperature could cause harm to you or the component. Please contact the
manufacturer if a storage temperature is surpassed.
Stimulator Receiver
Storage Temperature
Wearable Antenna Assembly
Storage Temperature
Medical tests and procedures –Before undergoing medical tests or
procedures, contact the clinician to determine if the procedure will cause
damage to the patient or to the System.
Physician instructions –Always follow the programs and therapy instructions
established by the clinician. Failure to do so may cause the therapy to be less
effective in providing pain relief.

English
Page | 12
Use the WAA as directed –Use the WAA only as explained by the clinician or as
discussed in the User Manual. Using the WAA in any other manner could result
in harm.
Do not dismantle the WAA–Do not dismantle or tamper with the device.
Tampering with the device could result in harm. If the device is not working
properly, contact the clinician for help.
Flammable or Explosive Environments –Do not use the WAA in flammable or
explosive environments. Using the WAA in one of these environments could
result in harm.
Use of another patient’s WAA - Never use another patient’s WAA. The therapy
programmed is a unique prescription for each patient. Use of another patient’s
WAA could result in overstimulation.
Activities requiring excessive twisting or stretching –Avoid activities that
potentially can put undue stress on the device. Activities that include sudden,
excessive, or repetitive bending, twisting, bouncing, or stretching can cause
your stimulator to fracture or migrate. This can result in a loss of stimulation,
intermittent stimulation, and additional medical procedures.
Scuba diving or hyperbaric chambers –Do not dive below 13 meters (45 feet)
of water or enter hyperbaric chambers above 1.5 atmospheres absolute (ATA).
These conditions can damage the device. Before diving or using a hyperbaric
chamber, discuss the effects of high pressure with the clinician.
Skydiving, skiing, or hiking in the mountains –High altitude should not affect
the System. However, take care to not put undue stress on the Stimulator.
During skydiving, the sudden jerking that occurs when the parachute opens can
dislodge or fracture the Stimulator. This can result in a loss of stimulation,
intermittent stimulation, and additional medical procedures.
Unexpected changes in stimulation –Electromagnetic interference, changes in
posture, and other activities can cause a perceived increase in stimulation.
Some patients have described this as a jolting or shocking sensation. You
should reduce your amplitude to the lowest setting and turn OFF your System
before engaging in activities that could become unsafe. Discuss these activities
with your clinician.

English
Page | 13
Interference during programming - If interference is suspected during
programming of the WAA, the clinician should confirm that the Bluetooth®
data transmission is operating properly. Bluetooth® data communication is
confirmed by the solid blue light on (when device is paired) and a fast blinking
yellow light (only when data is transmitted). If during the programming session
the light indicator is not blinking then the clinician should do the following:
•Terminate the current programming session and shut down the
WaveCrest Application.
•Check for sources of Bluetooth® interference in the surrounding area.
•Remove or turn off the source of interference.
•Re-establish the Bluetooth® link with the WAA through pairing.
•Resume programming by opening the WaveCrest Application.
•Confirm the light indicator is now blinking.

English
Page | 14
ADVERSE EVENT SUMMARY
Implantation of a spinal cord stimulation system is similar to any surgical
procedure. Risks include the following:
•Allergic or immune system response to implanted material
•Infection
•Leakage of cerebrospinal fluid
•Epidural hemorrhage, hematoma, or paralysis
Therapeutic use of the Freedom SCS System incurs the following risks:
•Undesired change in stimulation, including uncomfortable chest wall
stimulation
•Stimulator migration, erosion through the skin, or fracture causing a
loss of therapeutic effect
•Electromagnetic interference causing a change in System performance
•Loss of therapeutic effect despite a functioning system
Adverse events that could occur with the Freedom SCS System:
•Stimulator migration, resulting in altered stimulation therapy that may
be uncomfortable
•Stimulator fracture, resulting in loss of stimulation
•Infection, resulting in tissue sensitivity, redness and swelling
Adverse effects of stimulation are usually mild and go away when stimulation is
turned off. Contact your clinician immediately if you experience any problems.
Over time there could be changes in the level of pain control. Contact your
clinician if you experience a change in stimulation that you believe is a result of
the Stimulator slipping from the implant site.
English
Page | 15
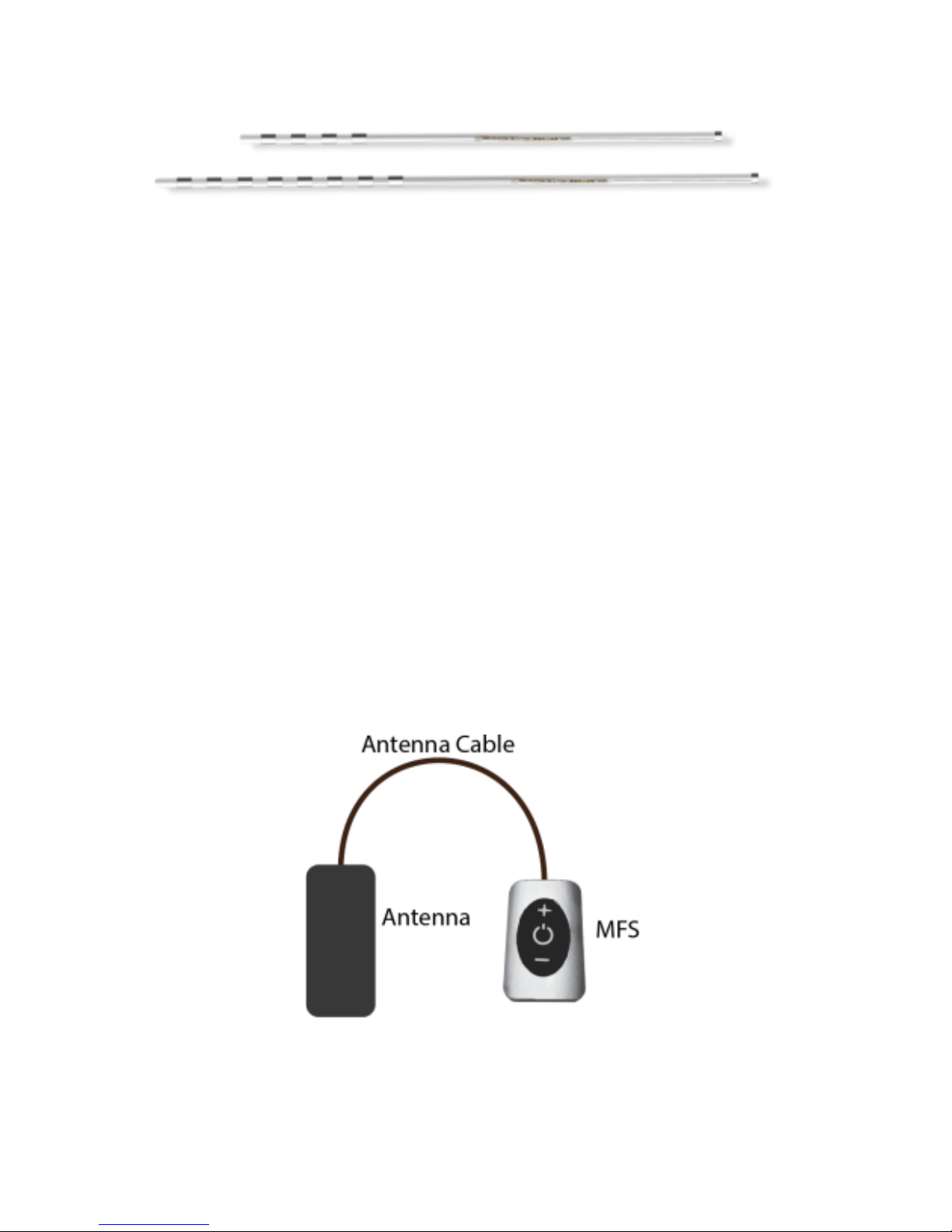
PARTS OF YOUR STIMULATION SYSTEM
Freedom Stimulator –Also known as an “implant” or “receiver”. The
Stimulator is a set of thin wires and a miniature receiver, covered with a
protective casing. The Stimulator has small metal electrodes near the tip that
are set to different electrical polarities. An electrical field of energy is created
when power is applied to the electrodes. The electrical field aids in blocking the
pain signals coming from certain nerves near the spinal column. The Stimulator
receives energy wirelessly from the external unit (WAA).
Wearable Antenna Assembly (WAA) –The WAA is an electronic device used to
power the Stimulator. The device contains a rechargeable battery with an
antenna and micro USB port (for charging only). The WAA is worn near the area
where the Stimulator-Receiver is implanted.
The WAA communicates with your Stimulator-Receiver by sending
radiofrequency signals through the antenna. Your Stimulator-Receiver only
accepts communication from your WAA. The clinician programs your WAA with
your specific Stimulation parameters from a wireless Bluetooth® programming
tablet.
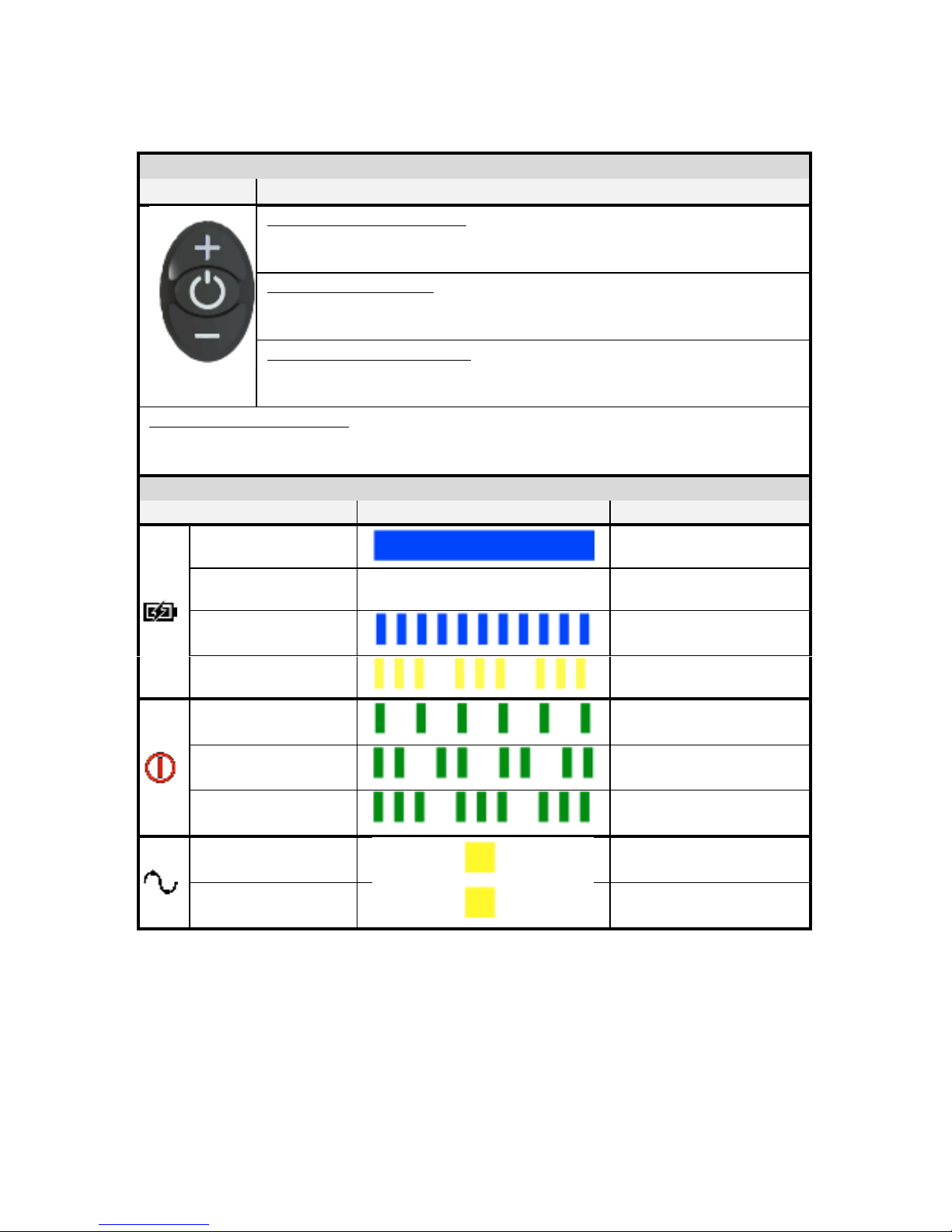
Antenna and Antenna Cable –The antenna sends out the wireless signal. The
antenna is permanently attached to the antenna cable. The antenna is
permanently connected to the Microwave Field Stimulator (MFS) with the
antenna cable.
English
Page | 16
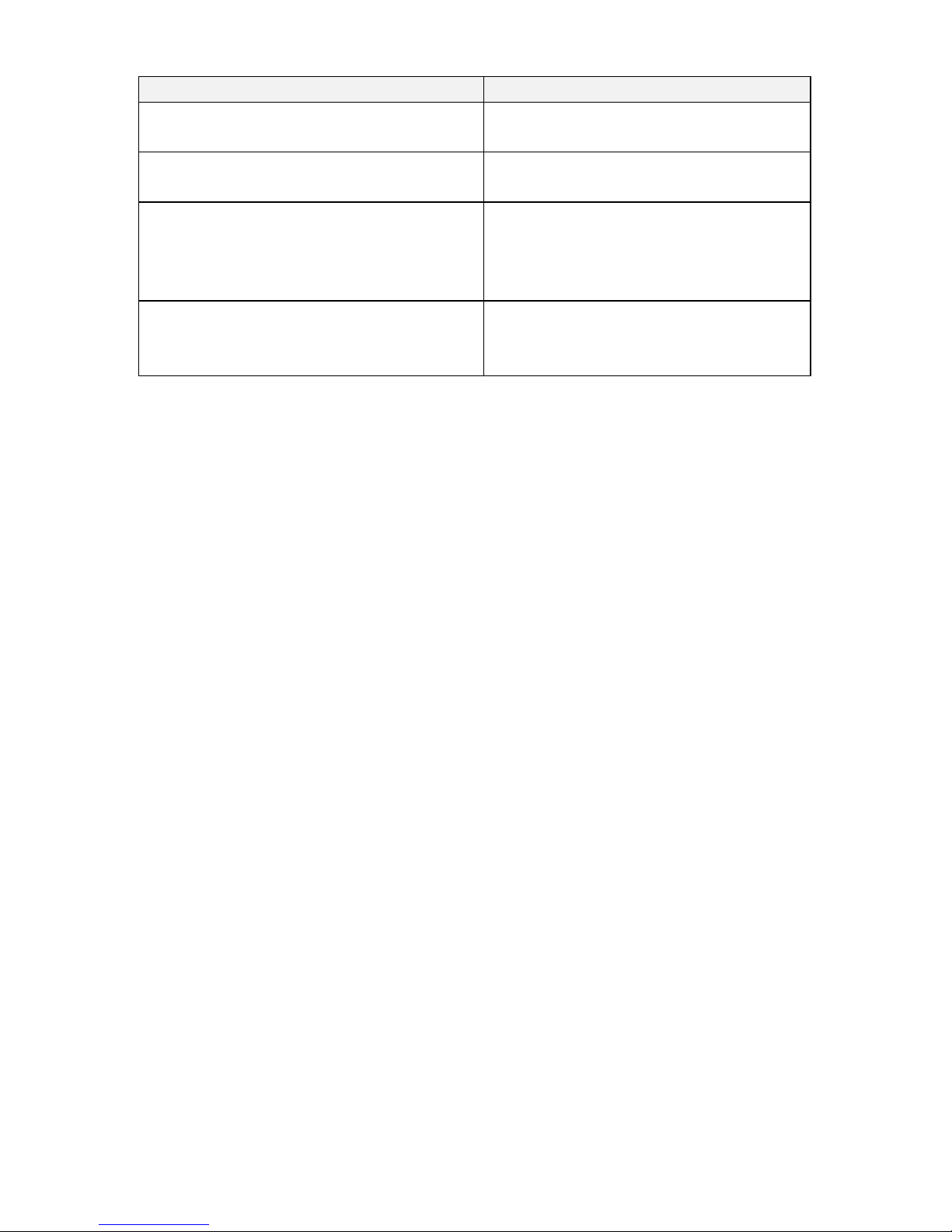
OVERVIEW OF THE USER CONTROLS
BUTTONS
Key
Action Description
Increase Amplitude Button –Used to increase the stimulation strength.
Holding the button down for more than 2 seconds ramps the amplitude
to the highest level, 24.
Power ON/OFF Button –Used to turn the WAA ON or OFF. Green LED is
a default indicator and will blink to indicate power status. A solid Yellow
light indicates that the antenna is disconnected and is not functioning.
Decrease Amplitude Button –Used to decrease the stimulation
strength. Holding the button down for more than 2 seconds ramps the
amplitude to the lowest level, 1.
Change Program Selection –Depressing the Increase Amplitude Button (‘+’) and the
Decrease Amplitude Button (‘-‘) at the same time will alternate the programmed setting
to either ‘1’ to ‘2’ to ‘3’ and back to “1’.
LEDS
Action Description
Pattern and Color
Description of Pattern
Charging
Solid Blue
OFF, Not Charging
None
Charge Complete
Battery FULL
Blinking Blue
Low Battery
Blinking Yellow
ON
Setting 1
Blinking Green (single)
ON
Setting 2
Blinking Green (double)
ON
Setting 3
Blinking Green (triple)
Minimum
Index
Blinks Once Yellow
Maximum
Index
Blinks Once Yellow

English
Page | 17
POSITIONING THE WAA
WARNING:
▪Do not place the WAA directly on your skin. Direct skin contact may
cause irritation and/or sensitivity to the materials. The WAA must be
placed overtop a thin layer of clothing at all times.
Steps:
1. The antenna must be placed over the general region of the Stimulator-
Receiver in order to transfer the optimal amount of energy.
2. The MFS and antenna cable does not need to be placed over the
Stimulator-Receiver. Your clinician will assist you to find the optimal
placement for effective therapeutic relief.

English
Page | 18
STARTING STIMULATION
Notes:
▪WAA automatically starts at lowest amplitude setting when turned on.
Steps:
1. Place the WAA Antenna directly over top of the Stimulator-Receiver.
2. Turn on the WAA by pressing the Power ON/OFF Key until the green
Power Indicator Light activates.
3. Adjust the amplitude as directed by your clinician by using the
Increase or Decrease Amplitude Key.
SELECTING A STIMULATION PROGRAM
The WAA has three program options that may be set. The green indicator light
is used to identify which program is currently active on the device.
•Program 1 = One Blink
•Program 2 = Two Blinks
•Program 3 = Three Blinks
Steps:
1. After powered “On”, the WAA defaults to the last program used.
2. Press “+” and “-” buttons at the same time to switch programs.
3. The light pattern will blink to signify which program is active.
INCREASING OR DECREASING AMPLITUDE
CAUTION:
Turn the power off or decrease the amplitude before changing the position of
the WAA Antenna to prevent possible uncomfortable stimulation.
Notes:
▪WAA must be turned on to increase or decrease the amplitude.
▪WAA does not need to be placed over the Stimulator to change
amplitude.
Steps:
1. Press the “Up” arrow key to increase the amplitude.
2. Press the “Down” arrow key to decrease the amplitude.
To receive the most effective therapy you will need to adjust your stimulation
throughout the day. Your clinician will provide guidelines about when you may
want to adjust your stimulation. The following table provides general guidelines
on how to adjust your stimulation.
English
Page | 19
Situation
Action
Stimulation is too strong
Decrease amplitude with the
Decrease Stimulation Key
Stimulation is not strong enough
Increase amplitude with the Increase
Stimulation Key
You have unexpected changes in
stimulation
1. Remove the WAA
2. Decrease amplitude
3. Put the WAA on your body
4. Adjust amplitude to desired level
You have tried adjusting stimulation
but are unable to find an effective
setting
Contact your clinician
MAINTENANCE
BATTERY CHARGING
Notes:
▪Use only the USB Charger and cable provided to perform charging.
▪The battery is built into the WAA and does not need to be removed.
Contact your clinician if you experience poor battery life.
▪You will need the USB Charger Kit to recharge the internal battery.
▪The antenna does not need to be connected during charging.
Steps:
1. Remove the WAA from your body.
2. Connect the USB cable to the power adapter.
3. Plug the power adapter into a wall outlet.
4. Connect opposite end of USB cable to the micro-USB port on the WAA.
5. Charging Indicator Light will stay solid on while the battery is charging.
6. Allow the battery to charge for at least four hours.
7. Charging Indicator Light will blink when the battery is fully charged.
8. WAA is now ready to be used again.

English
Page | 20
Common Questions
Response
How long will it take to
recharge a “dead” or
depleted battery?
It normally takes an average of three (3)
hours to recharge the battery.
When is the battery near
depletion, and how will I
know?
The yellow indicator light will begin
blinking. Eventually the WAA will turn off
and not respond to user controls. You
should connect the WAA to the charger as
illustrated above.
What happens if I deplete
the battery completely?
You cannot damage the WAA by running
the battery completely empty. The device
has safeguards to prevent this from
harming the battery. Connect the WAA to
the charger as described.
How long will a fully charged
battery provide power?
Eight (8) hours on average. The battery
performance is affected by the amount of
total power used on average.
When is the battery done
charging?
The battery is done charging when the
Charging Indicator Light (blue) blinks on/off.
Can I charge while the device
is turned on?
No, you cannot charge the device while it is
turned on. The stimulation cannot be used
while the device is connected the charger.
Must I disconnect the device
from the charger when full?
It is not necessary to disconnect the device
from the charger when full.
Will the WAA feel warm
during or after charging?
The WAA may feel warm during or
immediately after charging.
This manual suits for next models
1
Table of contents