STORZ MEDICAL MASTERPULS MP100 User manual

Operating Manual
MASTERPULS®MP100
Published: July 2015
Original language: German
Publisher:
STORZ MEDICAL AG
Lohstampfestr. 8
CH-8274 Tägerwilen
Switzerland
24523
0S.####
Part No. 23232.0100
19 300 02 0715
2
Table of Contents
Table of Contents
1 General Safety Information 6
1.1 Instructions for safe use 6
1.1.1 Designated use and operational safety. . . . . . . . . . . . . 6
1.1.2 Safety during treatment of the patient. . . . . . . . . . . . . 7
1.2 Warning against damage to equipment and the device 7
2 Principles 9
2.1 Physical principles 9
2.1.1 Indications . . . . . . . . . . . . . . . . . . . . . 9
2.1.2 Contraindications . . . . . . . . . . . . . . . . . . . 9
2.1.3 Side effects . . . . . . . . . . . . . . . . . . . . . 10
2.2 Preconditions for operation 10
2.2.1 Operating personnel . . . . . . . . . . . . . . . . . . 10
2.2.2 Training of the operator . . . . . . . . . . . . . . . . . 11
3 System Description 12
3.1 Control and functional elements 12
3.2 Scope of supply 13
3.3 Unpacking 13
3.4 Installation Instructions 14
3.4.1 Mounting the handpiece holder . . . . . . . . . . . . . . 14
3.4.2 Connecting the electrical power supply . . . . . . . . . . . . 14
3.4.3 Connecting handpiece . . . . . . . . . . . . . . . . . 15
3.4.4 Connecting tablet . . . . . . . . . . . . . . . . . . . 16
3.5 Compatibility 16
4 Operation 17
4.1 Switching on and off 17
4.2 Options for operation 17
4.3 Operation of the handpiece 18
4.4 Symbols and displays 19
4.4.1 Module selection . . . . . . . . . . . . . . . . . . . 20
4.4.2 Parameter selection and counter readings. . . . . . . . . . . . 21
4.4.3 Contact intensity and Skin Touch display . . . . . . . . . . . . 22
19 300 02 0715
3
Table of Contents
4.4.4 Treatment menu bar . . . . . . . . . . . . . . . . . . 24
4.4.5 Device info and settings menu bar . . . . . . . . . . . . . . 25
4.5 Touch screen operation 26
4.5.1 Set and reset device type . . . . . . . . . . . . . . . . . 26
4.5.2 Password protection . . . . . . . . . . . . . . . . . . 27
4.5.3 Setting brightness and volume . . . . . . . . . . . . . . . 29
4.5.4 Selecting the operating mode . . . . . . . . . . . . . . . 29
4.5.5 Selecting treatment parameters . . . . . . . . . . . . . . . 30
4.5.6 Loading indications . . . . . . . . . . . . . . . . . . 30
4.5.7 Saving indications . . . . . . . . . . . . . . . . . . . 34
4.5.8 Copying indications . . . . . . . . . . . . . . . . . . 35
4.5.9 Deleting an indication. . . . . . . . . . . . . . . . . . 36
4.5.10 Editing indications . . . . . . . . . . . . . . . . . . . 36
4.5.10.1 Storing treatment notes . . . . . . . . . . . . . . . . . 37
4.5.10.2 Loading images and/or videos . . . . . . . . . . . . . . . 37
4.5.10.3 Creating,deleting or editing treatment steps. . . . . . . . . . . 38
4.5.11 Patient treatment report . . . . . . . . . . . . . . . . . 39
4.5.11.1 Loading patient data . . . . . . . . . . . . . . . . . . 39
4.5.11.2 Editing patient data . . . . . . . . . . . . . . . . . . 41
4.5.11.3 Load treatment parameters . . . . . . . . . . . . . . . . 41
4.5.12 Creating new patient data . . . . . . . . . . . . . . . . 42
4.5.13 Exporting treatment data. . . . . . . . . . . . . . . . . 43
4.5.14 Deleting patient datasets . . . . . . . . . . . . . . . . . 43
4.5.15 Resetting the treatment shock counter . . . . . . . . . . . . 43
4.5.16 Software updates . . . . . . . . . . . . . . . . . . . 44
4.5.17 Changing software settings . . . . . . . . . . . . . . . . 44
4.5.18 Visible Body - Anatomy Atlas . . . . . . . . . . . . . . . 45
4.5.18.1 Starting Visible Body . . . . . . . . . . . . . . . . . . 45
4.5.18.2 Marking treatment regions . . . . . . . . . . . . . . . . 46
4.5.18.3 Exiting Visible Body . . . . . . . . . . . . . . . . . . 47
4.6 Setting treatment parameters 48
4.7 Start-up 48
4.8 Functional checks 49
4.9 Standard settings 49
4.10 Treatment 50
4.10.1 Setting parameters . . . . . . . . . . . . . . . . . . 51
4.10.2 Coupling the handpiece . . . . . . . . . . . . . . . . . 51
4.10.3 Triggering shocks . . . . . . . . . . . . . . . . . . . 51
4.10.4 Functions overview of the handpiece R-SW . . . . . . . . . . . 51
19 300 02 0715
4
Table of Contents
5 Cleaning, Maintenance, Overhaul 52
5.1 Cleaning 52
5.1.5 Cleaning the tablet . . . . . . . . . . . . . . . . . . 52
5.1.1 Cleaning of the handpieces . . . . . . . . . . . . . . . . 52
5.1.2 Fuse replacement . . . . . . . . . . . . . . . . . . . 53
5.2 Maintenance and safety checks 53
5.3 Disposal 54
5.4 Repair 54
5.5 Service life 54
6 Accessories 55
7 TechnicalSpecications 56
7.1 TechnicalSpecications 56
7.2 Type plate MASTERPULS®MP100 57
7.3 Conformity with directives 57
7.4 Conformity with standards 57
7.4.1 EMC guidelines and manufacturer’s declaration . . . . . . . . . . 58
7.5 Certicates 62
7.6 Symbols and labels 63
8 Warranty and Service 64
8.1 Warranty for the control device 64
8.2 Warranty for the handpiece 64
8.3 Service 64
19 300 02 0715
5
Preface
Preface
Warning notes
This manual contains warnings, safety instructions and specic operating instructions
in accordance with liability regulations.
DANGER refers to a situation of acute danger which, if not avoided, could lead to
serious or fatal injury.
DANGER!
The source of the danger is stated here.
These are the possible consequences!
• The instructions for avoiding the danger are given here.
WARNING refers to a situation of potential danger which, if not avoided, could lead
to serious injury.
WARNING!
The source of the danger is stated here.
These are the possible consequences!
• The instructions for avoiding the danger are given here.
CAUTION indicates that incorrect operation could lead to minor injuries.
CAUTION!
The source of the danger is stated here.
These are the possible consequences!
• The instructions for avoiding the danger are given here.
ATTENTION indicates that incorrect operation could lead to damage to the device.
ATTENTION!
The source of the danger is stated here.
These are the possible consequences!
• The instructions for avoiding the danger are given here.
Other instructions
NOTE
Additional information concerning specic features or operating instructions is
preceded by the term 'NOTE'.
19 300 02 0715
6
General Safety Information
1 General Safety Information
1.1 Instructions for safe use
The following chapter contains all safety information that has to be followed when
working with the MASTERPULS®MP100.
WARNING!
Incorrect handling of the device.
Possibility of injuries to the patient and the operating personnel!
• Read this chapter carefully before you start using the MASTERPULS®
MP100.
• Read the separate operating manuals for all devices associated
with the MASTERPULS®MP100.
1.1.1 Designated use and operational safety
In order for the user to use this device in accordance with its designated use, the user
must possess the necessary technical prociency, and knowledge of the operating
manual.
The device is only allowed to be used for the applications described in Chapter 2.1.1
indiCatiOns.
• Only perform treatments approved by STORZ MEDICAL AG!
Furthermore, the device is only allowed to be operated by trained personnel who
comply with the preCOnditiOns fOr OperatiOn in Chapter 2.2.
All status and error messages signaled during treatment must always be attended to
without delay.
Checks and inspections prior to treatment
Before using the device, the user must make sure it is functioning safely and that it is
in proper condition.
• It is essential to perform the functional checks after switching on the MASTERPULS®
MP100 before starting treatment. Read about this in Chapter 4.8 funCtiOnal CheCks.
• Have the maintenance procedures recommended by the manufacturer carried out by
authorised personnel (see also Chapter 5.2 MaintenanCe and safety CheCks).
Protection against electrical hazard
Sources of voltage can give rise to currents as a result of body resistance which not
only ow through the patient but can also impair or even endanger the physician and
the nursing staff.
• Devices which are not medical products in accordance with EN 60601 must be set
up outside the vicinity of the patient.
• Do not touch electrical connectors while you are touching the patient.
19 300 02 0715
7
General Safety Information
• Disconnect the MASTERPULS®MP100 from the mains before starting any cleaning
or maintenance work!
• Disconnect the connected handpieces from the device before carrying out cleaning
and maintenance work. Do not reconnect them until they have been completely
reassembled!
Protection against noise
The noise level during administration of shock waves is within the safe area.
Nevertheless, we recommend wearing suitable ear protection during treatment in
order to minimise exposure to noise.
1.1.2 Safety during treatment of the patient
General note:
Organs with gas inclusions, in particular parts of the lung, are NOT allowed to be
exposed to shock waves.
As it passes through tissue, the shock wave’s energy is slightly reduced; this reduction
is signicantly weakened by the bone structure.
Shock waves can give rise to undesirable heart reactions. The patient must be
continuously observed during the treatment.
Only perform treatments approved by STORZ MEDICAL AG!
The user is responsible for correctly positioning the handpieces and correctly selecting
the treatment zone.
No more than 6,000 shock waves are allowed to be administered without
interruption.
1.2 Warning against damage to equipment and
the device
Any damage to the device resulting from incorrect operation is not covered by the
manufacturer’s warranty.
Electromagnetic compatibility
This device complies with the requirements of the applicable standard on
electromagnetic compatibility.
Nevertheless, portable and mobile HF communications equipment (e.g. mobile
phones) can interfere with medical electrical equipment.
This device is subjected to special precautions regarding EMC and needs to
be installed according the EMC guidelines in Chapter 7.4.1 eMC guidelines and
ManufaCturer’sdeClaratiOn.
The use of accessories or cables that are not authorised by the manufacturer can
result in increased interference emissions or reduced resistance to interference
emissions by the device.
19 300 02 0715
8
General Safety Information
The MASTERPULS®MP100 is not allowed to be positioned immediately next to
or jointly with other devices. If the operation near or jointly with other devices is
required, the MASTERPULS®MP100 must be tested in that particular environment to
ensure operation according to technical specication.
The system must only be connected to properly earthed and correctly installed
shockproof sockets!
Set-up and operation
There are ventilation slits on the side of the device which must not be covered by
other objects.
• Check that the system is in perfect working order before each use. Read about this in
Chapter 4.8 funCtiOnal CheCks.
• Never cover the device when in use!
• Make absolutely sure that no liquid can seep into the system housing or
handpiece.
Storage and transport
Incorrect storage and transport can result in damage to the device and device failure.
• Make sure that no cables are crushed or sheared.
Disposal
• Comply with national disposal regulations when disposing of the MASTERPULS®
MP100 or individual components.
• Comply with the relevant information in the operating manuals for the additional
devices.
19 300 02 0715
9
Principles
2 Principles
2.1 Physical principles
The MASTERPULS®MP100 is a compressed air–operated ballistic shock wave genera-
tor. The shock waves in the MASTERPULS®MP100 are generated with a precision
ballistic mechanism in the handpiece. A projectile is accelerated by compressed air.
The motion and weight of the projectile produce kinetic energy. When the projectile
impacts against an immovable surface, the shock transmitter, this kinetic energy is
converted into sound energy. This acoustic pulse is transmitted into the tissue to be
treated either directly or via an acoustic impedance adapter with the help of a gel.
Physically speaking, these are radial pressure waves. The applied pressure pulse propa-
gates radially within the tissue and has a therapeutic effect on areas of the tissue near
the surface, in particular.
NOTE
Medical devices operating on the basis of the above principle are generally refer-
red to as radial shock wave systems in modern medical literature.
2.1.1 Indications
– Calcaneal spur /plantar fasciitis
– Shoulder pain with or without calcications
– Achillodynia
– Trochanteric bursitis / proximal iliotibial band friction syndrome
– Radial/ulnar humeral epicondylitis
– Patellar tip syndrome
– Tibial edge syndrome
– Insertion tendonitis in general
– Treatment of deep muscle trigger points
– Treatment of supercial muscle trigger points, myofascial trigger points
– Supercial insertion tendonitis (paratendinary area)
– Chronic back pain (cervical/lumbar parts of vertebral column)
2.1.2 Contraindications
CAUTION!
No claims are made regarding the completeness or unlimited validity
of this list of contraindications.
19 300 02 0715
10
Principles
Treatment with the STORZ MEDICAL MASTERPULS®MP100 is not permitted in the
following cases:
– Coagulation disorders (haemophilia)
– Use of anticoagulants, especially Marcumar
– Thrombosis
– Tumour diseases, carcinoma patients
– Pregnancy
– Epiphyseal fusion areas in children
– Cortisone therapy up to 6 weeks before rst treatment
CAUTION!
Shock waves must not be applied to target areas located above air
lled tissue (lungs), nor to any regions near large nerves, vessels,
the spinal column or head (apart from the face).
2.1.3 Side effects
Treatment with the MASTERPULS®MP100 may cause the following side effects:
– Swelling, reddening, haematomas
– Petechiae
– Pain
– Skin lesions after previous cortisone therapy
These side effects generally abate after 5 to 10 days.
2.2 Preconditions for operation
2.2.1 Operating personnel
The MASTERPULS®MP100 is intended exclusively for use by medical specialists and
may only be used by suitably qualied and trained medical personnel.
Such a specialist is expected to have practical knowledge of medical procedures and
applications as well as of the technology, and should be experienced in treating the
indications stated in Chapter 2.1.1 indiCatiOns.
Users must have basic physical and cognitive abilities such as vision, hearing and
literacy, and have basic functional use of their upper extremities.
The device is designed for a demographic target group between 18 and 65 years.
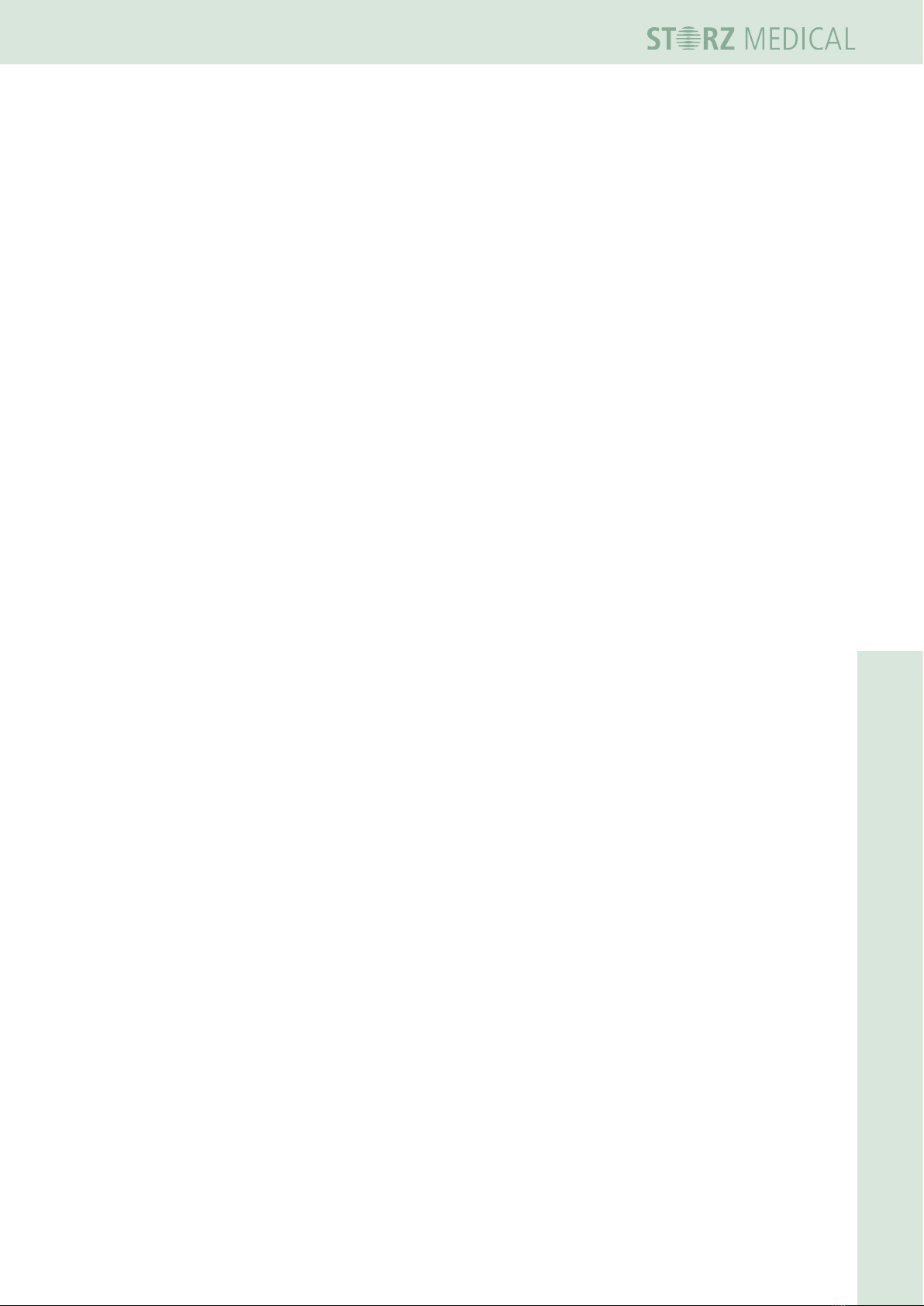
19 300 02 0715
11
Principles
2.2.2 Training of the operator
Operators of the MASTERPULS®MP100 must have been adequately trained in
using this system safely and efciently before they operate the device described in
this handbook. An introduction to the principles of operation will be provided by
your STORZ MEDICAL dealer with reference to this operating manual and will be
documented in the system logbook.
The operator must be instructed in the following points:
– Instruction in the operation and designated use of the device with practical
exercises
– Mechanism of action and function of the device and the energies delivered by it
– All component settings
– Indications for use of the device
– Contraindications and side effects of the therapy waves
– Explanation of the warnings in all operating modes
– Instruction in how to perform the functional checks
Further training requirements vary from country to country. It is the operator’s
responsibility to ensure that the training meets the requirements of all applicable
local laws and regulations. Further information about training in the operation of this
system can be obtained from your STORZ MEDICAL dealer. However, you can also
contact the following address directly:
STORZ MEDICAL AG Telephone: +41 (0) 71 677 45 45
Lohstampfestrasse 8 Fax: +41 (0) 71 677 45 05
Postfach
CH-8274 Tägerwilen
Switzerland

19 300 02 0715
12
System Description
3 System Description
3.1 Control and functional elements
The MASTERPULS®MP100 is controlled using the operating and display elements on
the handpiece as well as using the SMAG tablet display.
1 main switch
1
Fig. 3-1 Front side of MASTERPULS®MP100
6
5
432
1
Fig. 3-2 Rear side MASTERPULS®MP100
1 Handpiece connector R-SW
2 Handpiece connector V-ACTOR
3 USB B 1.1 Device interface
4 USB A 1.1 Host interface
5 Mains connector
6 Mains Fuse holder
NOTE
The USB connection (Fig. 3-2/3) is generally used for service purposes.
In addition it is possible to connect a Tablet PC.
The USB connection (Fig. 3-2/4) is only used for connecting a USB memory stick
for software update which supports the USB V1.1 protocol or higher.
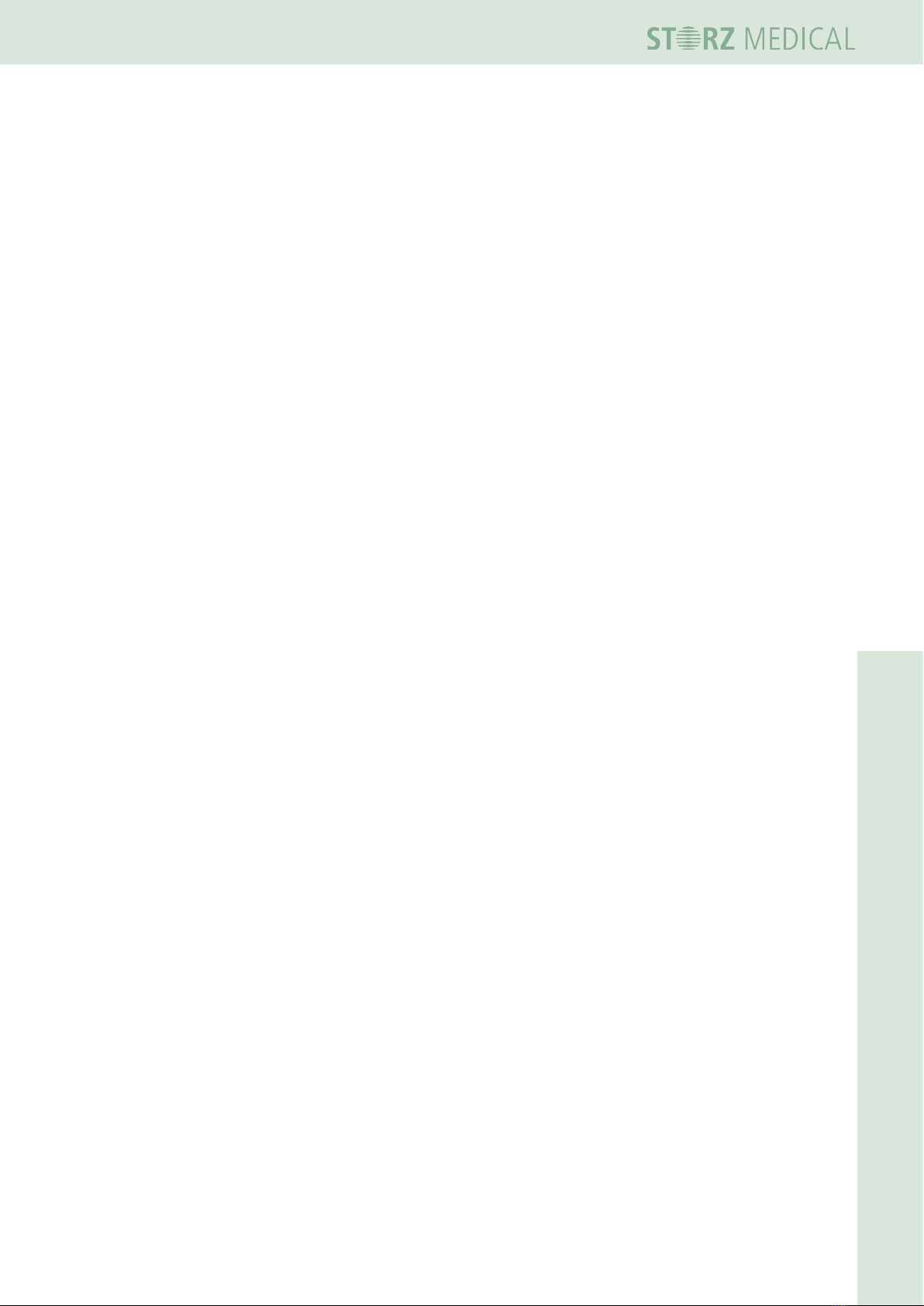
19 300 02 0715
13
System Description
3.2 Scope of supply
The standard scope of supply of the STORZ MEDICAL MASTERPULS®MP100 includes
the following items:
– MASTERPULS®MP100 control device
– Mains cable (EU / USA)
– Gel bottle
– User manual (operating manual, system logbook and training records)
– R-SW handpiece set
– Handpiece holder
3.3 Unpacking
• Carefully remove the instrument and accessories from the packaging container.
• Check that all items are included in the packaging container and that they are not
damaged.
• Contact your supplier or the manufacturer immediately if any items are missing or
damaged.
• Retain the original packaging. It may prove useful for any later equipment trans-
port.
19 300 02 0715
14
System Description
3.4 Installation Instructions
3.4.1 Mounting the handpiece holder
There are two different handpiece holder:
– for handpieces R-SW
– for handpieces V-ACTOR.
1
1 Openings for insertion of
the handpiece holder
Fig. 3-3 Mounting the handpiece holder
The mounting of the handpiece holder is equal for both models.
• Push the holder into the provided openings on the Masterpuls®MP100.
– There are openings for 4 handpiece holders -2 on the right side and two on the
left side of the MP100.
3.4.2 Connecting the electrical power supply
• Connect the supplied mains cable to the mains connector on the rear side of the
device.
1
1 Mains connector
Fig. 3-4 Connecting the electrical power supply
• insert the mains cable into the socket.

19 300 02 0715
15
System Description
ATTENTION !
When setting up the instrument, make sure that the air outlets on the housing of
the MASTERPULS®MP100 are not blocked.
The instrument must only be connected to properly earthed and correctly installed
shockproof sockets!
The device must be positioned in a way so that disconnection from the mains is
easy to do.
3.4.3 Connecting handpiece
• Insert the plug of the handpiece into the corresponding handpiece connector on
the left rear side of the device.
1 Handpiece connector R-SW
2 Handpiece connector V-ACTOR
1
2
Fig. 3-5 Handpiece connectors
• Make sure that the red dot on the socket is aligned with the red dot on the
handpiece connector.
• Place the handpiece into the handpiece holder.
NOTE
Please also refer to the separate operating manual for your handpiece.

19 300 02 0715
16
Operation
3.4.4 Connecting tablet
1
1 USB connector to MP100
2 mains switch of the tablet
2
Fig. 3-6 Tablet with power supply and USB cable
1 USB connector for
tablet display
1
Fig. 3-7 Connection of the tablet - rear side of the MP100
• Connect the USB connector of the tablet in the USB connector on the rear side of
the MP100.
• Press the mains switch of the tablet on the upper side of the tablet.
NOTE
In order to operate the MASTERPULS MP100 in this conguration, ensure that
the software of the control device corresponds version 24040.02 or higher
and
the software of the handpiece corresponds version 24264.03 or higher.
3.5 Compatibility
The STORZ MEDICAL Masterpuls MP100 is allowed to be operated with the following
handpieces:
– handpiece R-SW part.no. 21700_xxxx
– handpiece R-SW part.no. 23213_xxxx

19 300 02 0715
17
Operation
4 Operation
4.1 Switching on and off
• Switch on the control device using the main switch on the front side of the device.
• Switch on the tablet on the upper left side.
1 mains switch with LED (lightening
during power on)
2 mains switch of the tablet
2
1
Fig. 4-1 Mains switch
NOTE
When switching on the tablet the software starts but treatment is not possible.
Make sure that both devices are switched on.
4.2 Options for operation
The following options for the use of MASTERPULS MP100 are available:
– over the display of the handpiece
– over the touch screen of the tablet
19 300 02 0715
18
Operation
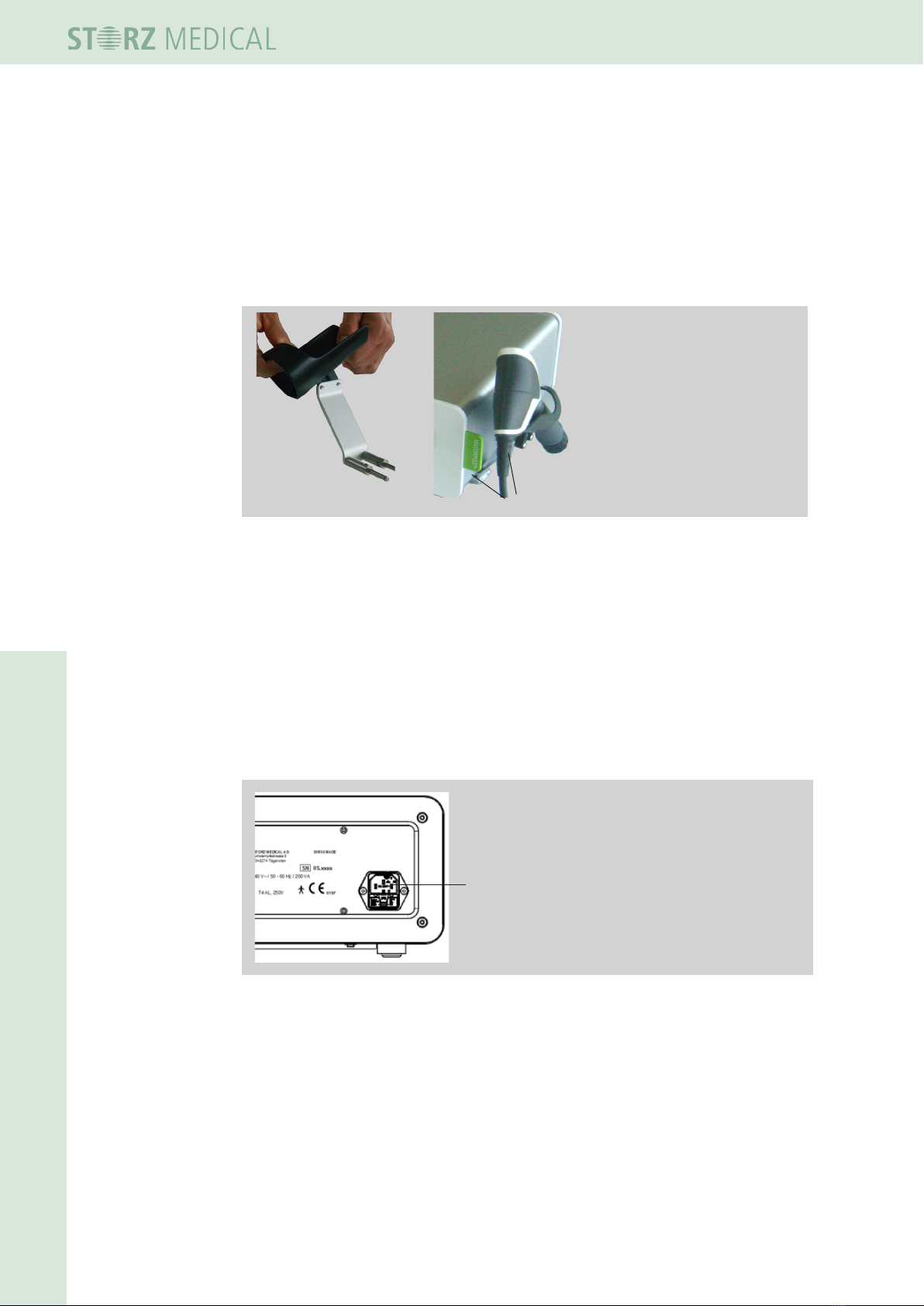
4.3 Operation of the handpiece
This device can be controlled directly using the handpiece. Corresponding setting
buttons can be used for selecting the treatment parameters. The indicator window
shows which setting has been selected.
12
6
5
4
3
10
9
8
7
11
1 decrease energy
2 increase energy
3 decrease frequency
4 increase frequency
5 Trigger button
6 Display energy
7 Set nominal shock
wave
8 Shock wave counter
9 Display frequency
10 key combination with
double function
11 key combination with
double function
(10 and 11 see separate
operating manual of the
handpiece )
Fig. 4-2 Display and setting buttons of the R-SW handpiece
For operation of the handpiece using the embedded display read the separate Opera-
ting Manual Of the r-sW handpieCe.
19 300 02 0715
19
Operation
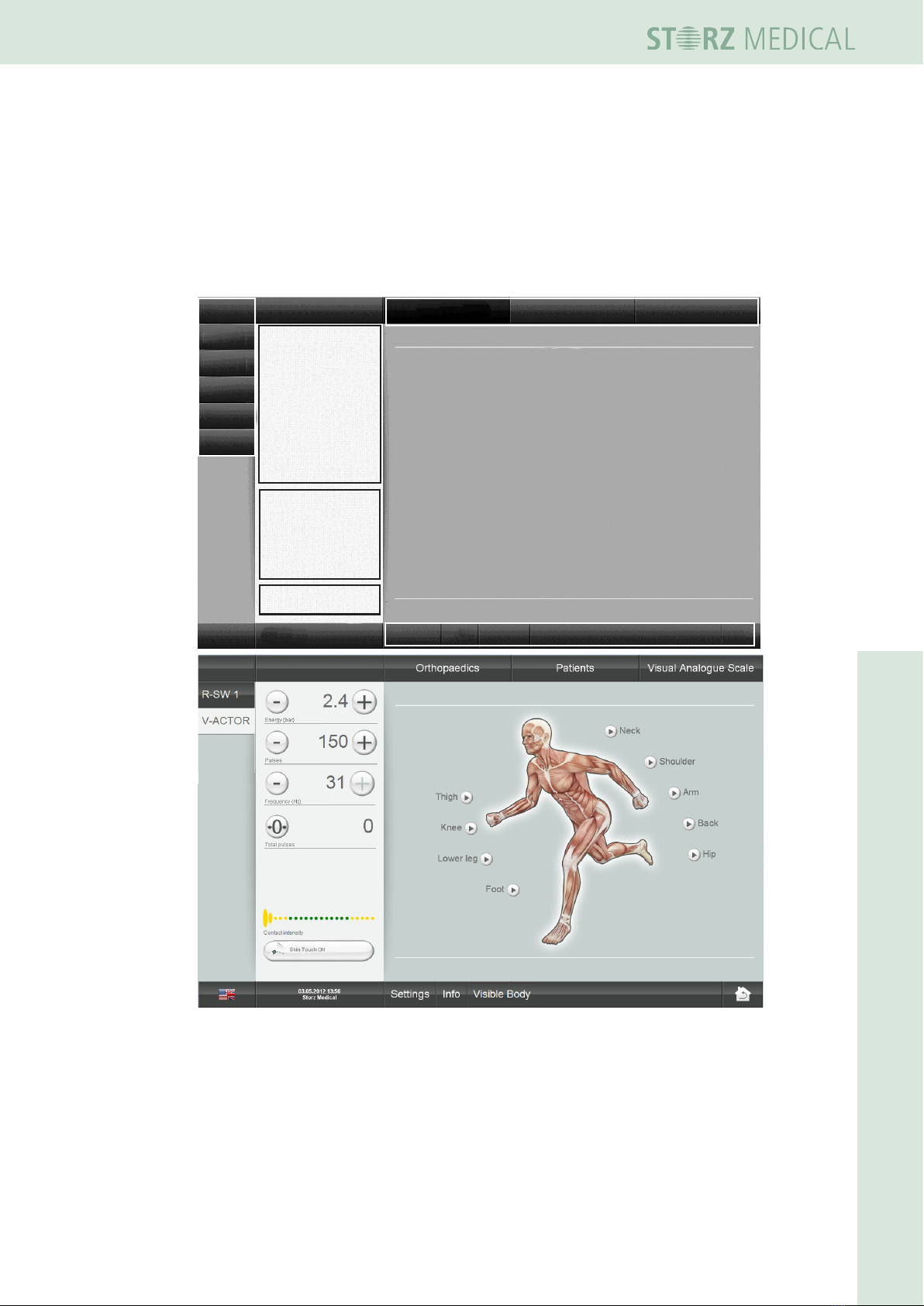
4.4 Symbols and displays
The MP100 can be optionally controlled by the optional tablet when using the R-SW
handpiece as well as the V-ACTOR handpiece. This allows also an individual adjust-
ment of the parameters for the V-ACTOR handpiece.
The user interface is divided into various areas:
Module selection
Parameter
selection
Counter
display
Contact
pressure
Menu bar: Monitor
Monitor
Menu bar: Info & settings
Language Date / time
Fig. 4-3 Structure of the user interface
19 300 02 0715
20
Operation
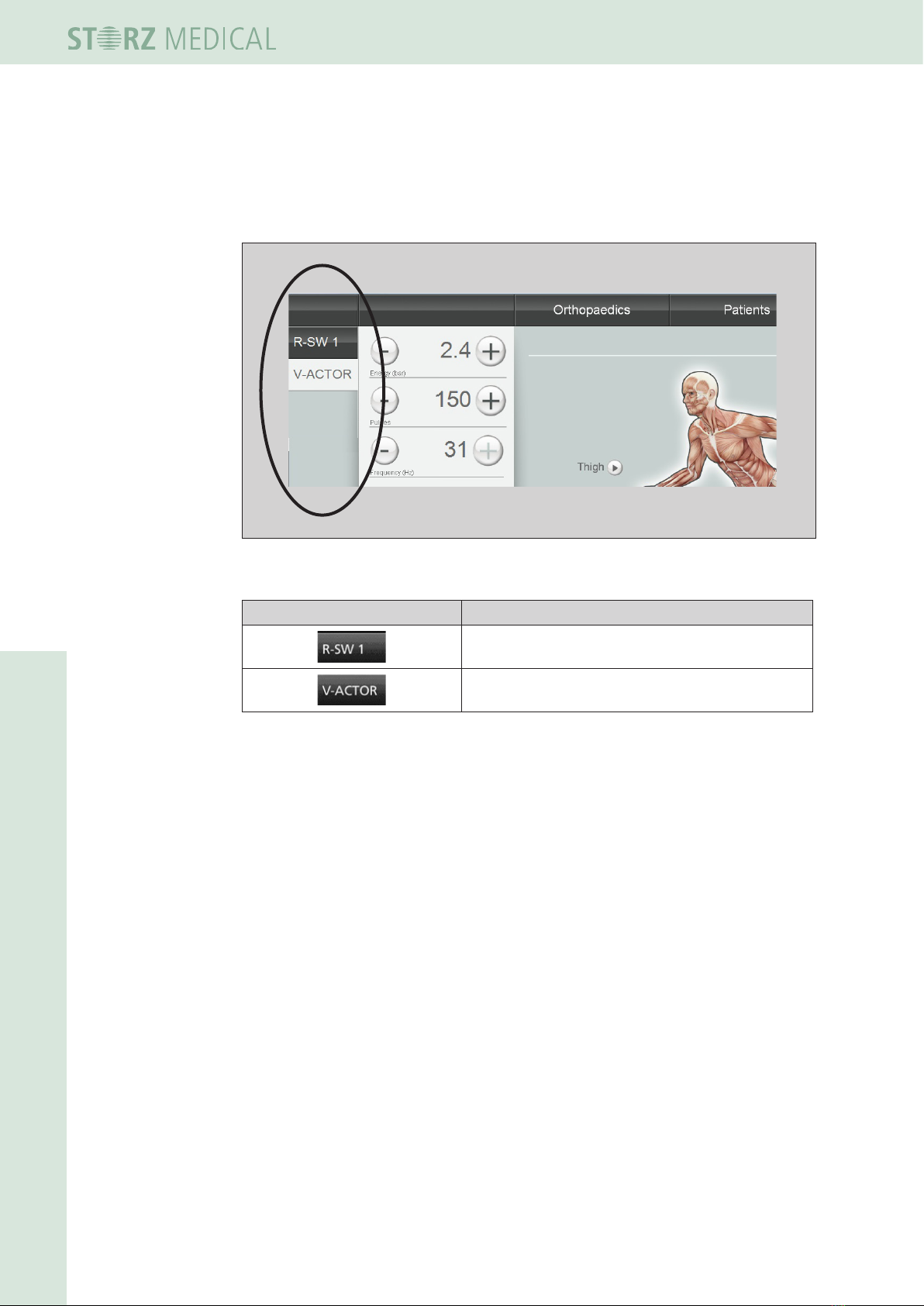
4.4.1 Module selection
The eld at the top left is used for displaying the operating modes that can be select-
ed. Once a handpiece is connected, it is possible to activate the corresponding operat-
ing mode in the module selection area. The active module button is highlighted.
Fig. 4-4 Module selection eld
Symbols Meaning
R-SW mode: Select handpiece 1
Vibration therapy: Select V-ACTOR
Tabelle 1-1 List of symbols for module selection
Table of contents