NeoMedLight BiliCocoon Nest System User manual

Instructions for Use / Manuel d’utilisation :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13
EN / FR
BiliCocoon™ System
Phototherapy System
Système de Photothérapie
BiliCocoon™Nest System
BiliCocoon™Bag System

Instructions for Use / Manuel d’utilisation :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13
EN / FR
Table of contents
User Responsibility...............................................................................................................................................1
1PRODUCT DESCRIPTION AND APPLICATION.................................................................................. 2
1.A Description ............................................................................................................................................ 2
1.B Intended use of the Device .................................................................................................................. 2
1.C Additional information ......................................................................................................................... 2
2SAFETY INFORMATION ....................................................................................................................... 2
Types of Safety Precautions .......................................................................................................................................2
3COMPONENTS / SUB-ASSEMBLIES AND USER CONTROLS ......................................................... 6
3.A System Description .............................................................................................................................. 6
3.B Light Box Features ............................................................................................................................... 9
Controls.......................................................................................................................................................................9
3.C Pad Technical Features...................................................................................................................... 10
3.D Technical Features of the Disposable.............................................................................................. 11
4INSTALLATION AND USE .................................................................................................................. 11
4.A Light Box Positioning......................................................................................................................... 11
4.BPad Setup ............................................................................................................................................ 12
4.C Verification of the Irradiance Value................................................................................................... 13
4.D Setup of the Disposable and Positioning of the Infant................................................................... 15
4.E Performing a Treatment ..................................................................................................................... 17
Precautions...............................................................................................................................................................17
Setting a Session ......................................................................................................................................................17
Resetting a Session or Changing the Settings of a Session .....................................................................................17
Interrupting a Session ...............................................................................................................................................17
Stopping the System.................................................................................................................................................18
5CARE AND MAINTENANCE ............................................................................................................... 18
5.A List of Authorized Cleaning Agents and Disinfectants................................................................... 18
5.B Light Box Cleaning ............................................................................................................................. 19
5.C Pad Cleaning & Dinsinfection............................................................................................................ 19
5.D No Cleaning for the Disposable ........................................................................................................ 20
5.E Failures and Possible Resolutions ................................................................................................... 20
5.F Maintenance Operations.................................................................................................................... 22
6HOME USE........................................................................................................................................... 22
RECOMMENDATIONS FOR CLINICIANS ...............................................................................................................22
RECOMMENDATIONS FOR THE HOME USER......................................................................................................22
7PRODUCT CATALOG NUMBERS...................................................................................................... 25
8TECHNICAL REFERENCES ............................................................................................................... 25
8.A BiliCoon™ System Spectral Emission ............................................................................................. 25
8.B Standards and regulations ................................................................................................................ 25
9WARRANTY......................................................................................................................................... 26
ANNEX A: Service Manual........................................................................................................................ 26
A.1 Purpose ..............................................................................................................................................................26
A.2 Maintenance Operations.....................................................................................................................................26
A.3 Calibration...........................................................................................................................................................27
A.4 Dust Filter Replacement .....................................................................................................................................28
A.5 Fuse Replacement..............................................................................................................................................29

Instructions for Use / Manuel d’utilisation :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13
EN / FR
Responsabilité de l’utilisateur............................................................................................................................31
1DESCRIPTION DU PRODUIT ET APPLICATION............................................................................... 32
1.A Description .......................................................................................................................................... 32
1.B Usage prévu du Dispositif ................................................................................................................. 32
1.C Informations supplémentaires .......................................................................................................... 32
2INFORMATIONS DE SECURITE......................................................................................................... 32
Types de consignes de sécurité................................................................................................................................32
3COMPOSANTS / SOUS-ENSEMBLES ET COMMANDES UTILISATEURS..................................... 38
3.A Description du Système..................................................................................................................... 38
3.B Caractéristiques de la Light Box....................................................................................................... 40
Commandes..............................................................................................................................................................41
3.C Caractéristiques techniques du Pad ................................................................................................ 42
3.D Caractéristiques techniques de l’usage unique .............................................................................. 42
4INSTALLATION ET MODE D’EMPLOI................................................................................................ 42
4.A Placement de la Light Box................................................................................................................. 43
4.B Mise en place du Pad ......................................................................................................................... 43
4.C Vérification de l’irradiance................................................................................................................. 44
4.D Mise en place de l’usage unique et installation du nourrisson ..................................................... 46
4.E Mise en route du traitement............................................................................................................... 48
Précautions...............................................................................................................................................................48
Programmer une session..........................................................................................................................................48
Changer la programmation d’une session en cours ou remettre la session à zéro ...................................................49
Interrompre une session............................................................................................................................................49
Arrêter le Dispositif....................................................................................................................................................49
5ENTRETIEN ET MAINTENANCE ........................................................................................................ 49
5.A Liste des agents nettoyants and désinfectants autorisés ............................................................. 50
5.B Nettoyage de la Light Box.................................................................................................................. 50
5.C Nettoyage & Désinfection du Pad ..................................................................................................... 51
5.D Pas de nettoyage pour l’usage unique ............................................................................................. 52
5.E Dysfonctionnements et résolutions possibles................................................................................ 52
5.F Opérations de maintenance............................................................................................................... 53
6USAGE A DOMICILE........................................................................................................................... 55
RECOMMANDATIONS POUR LES PROFESSIONNELS DE SANTE .....................................................................55
RECOMMANDATIONS POUR LES UTILISATEURS A DOMICILE..........................................................................55
7REFERENCES PRODUITS.................................................................................................................. 56
8REFERENCES TECHNIQUES............................................................................................................. 57
8.A Emission spectrale du Système BiliCocoon™ ................................................................................ 57
8.B Normes et règlementations ............................................................................................................... 57
9GARANTIE ........................................................................................................................................... 57
ANNEX A: Fiche d’intervention ................................................................................................................ 58
A.1 Objectif................................................................................................................................................................58
A.2 Operations de Maintenance................................................................................................................................58
A.3 Procédure de Calibration....................................................................................................................................59
A.4 Remplacement du filtre à poussière....................................................................................................................60
A.5 Remplacement du fusible ...................................................................................................................................61

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 1
EN
NeoMedLight
88-90 rue Frédéric Faÿs 69100 VILLEURBANNE, FRANCE
October 2017
User Responsibility
These Instructions for Use describe the proper setup, use, and maintenance of the BiliCocoon™ phototherapy
system
a
- the Device or the System.
The Device is to be used exclusively by a properly trained user and should not be used if it is damaged,
contaminated, or if parts are missing. Instead, please contact the supplier immediately. For questions about
Device care and maintenance please contact the supplier or authorized staff member of your facility.
The user is solely responsible for the risks to the patient, clinicians, third parties or properties or for a treatment
with inadequate performances due to an abusive or improper use, inadequate maintenance, reparation or
modifications made by unauthorized individuals.
Any serious incident which occurs in relation to the device should be reported to the manufacturer and the local
competent authority of the state in which the user and/or patient is established. You can contact NeoMedLight
at the following address: quality@neomedlight.com.
According to the US federal law the Device sale is restricted to licensed medical practitioners or clinicians or
under their approval.
NeoMedLight declares that the Device complies with the European Directive 93/42/EEC.
The CE mark was obtained and the Device was launched in 2016.
This product is to be handled with care and to be processed separately from consumer
waste. Waste of Electrical and Electronic Equipment (WEEE) can pollute the environment
and the product is to be disposed of according to its specific Directive 2012/19/EU and
following the appropriate paths.
Contact the local authorities or the supplier to determine the proper method of disposal of
potentially biohazardous parts and accessories.
a
The term “System” is not used in this document with the meaning given by the standard EN 60601-1, definition 3.64. It stands for the group
of interconnected parts which compose the medical device.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 2
EN
1 PRODUCT DESCRIPTION AND APPLICATION
1.A Description
The BiliCocoon™ System is a phototherapy system designed to treat unconjugated hyperbilirubinemia in the
infant. It is a phototherapy device as defined by EN 60601-2-50, which emits light in the absorption spectrum of
the bilirubin, from 430 to 490 nm
b
, thus reducing the bilirubin concentration in the body of infants.
1.B Intended use of the Device
The BiliCocoonTM Phototherapy System is intended for the treatment of unconjugated hyperbilirubinemia
c
; in
the population of neonates and infants under 3 months old and weighing less than 10kg. It can be used in the
clinical setting or in the home.
1.C Additional information
- Only for the treatment of unconjugated hyperbilirubinemia, which is responsible for the majority of cases
of neonatal jaundice.
- Only with medical prescription
- Only for the duration indicated in the treatment protocol
- Target patient population: neonates and infants
d
.
- Over the patient's entire body except for genitals and eyes –eye protection must be worn.
- To be used with a single patient
- To be used in hospital or at home: adapted to different environments for use (in an incubator, on a table,
in a cradle, in the arms of a parent or caregiver).
- Not to be used on damaged skin
2 SAFETY INFORMATION
Types of Safety Precautions
Warning
It informs of a danger or a hazard for the infant or the operator.
Important
It mentions the actions required to obtain the expected clinical results.
b
96% of light emission is in this wavelength range.
c
The Device is not designed to treat cholestatic jaundice.
d
Under 3 months old and weighing less than 10 kg –definition according to EN 60601-2-50. The term “infant” used in this
document is used in compliance with this definition according to EN 60601-2-50.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 3
EN
Caution
It indicates a risk of damage to the Device
Warning
Prerequisites
The BiliCocoon™ phototherapy system must only be used by clinicians who are qualified and
aware of the treatment- and device-associated risks and benefits; or by laypersons who are
properly trained and supervised by these clinicians.
Home Use: see section 6 of these Instructions for Use.
The BiliCocoon™ phototherapy device is not to be used for other medical indications or on
different body parts than those recommended. Improper use may lead to an ineffective
treatment (i.e. no benefits) or to potential risks to the patient's health (adverse events).
Warning
Duration of Session:
Use the device only for the time prescribed. A use that does not take into account the treatment
plan may lead to an ineffective treatment or to potential risks to the patient's health
Warning
No modification of the BiliCocoon™ Phototherapy System is allowed. Do not use any
accessories, detachable parts and materials not described in these instructions for use.
The use of a System which has been modified in terms of performances or by replacing its
parts disregarding the manufacturer’s indications may lead to risks to the patient (e.g. adverse
events), the user, other peoples or properties.
Warning
Intensive phototherapy (>30 μW∙cm-2∙nm-1) may not be adapted to all infants (e.g. preterm
infants with a weight ≤1000 g)
Warning
Blocking the light source or reducing the exposed body surface should be avoided
Warning
Contraindications
The BiliCocoon™ phototherapy device must not be used:
oOn patients with congenital porphyria
oOn patients with family history of porphyria
oIn conjunction with drugs or agents that are photosensitizers
oOn patients requiring a sterile environment or equipment and/or who present with
skin lesions.
A use that does not take into account these contraindications may lead to an ineffective
treatment (i.e. no benefits) or to potential risks to the patient's health (e.g. adverse events).
Warning
Eye Protection
Prolonged exposure to phototherapy light may cause damage to the eyes. The use of the
BiliCocoon™Phototherapy System without suitable protection of the infant’s eyes may lead to
risks to the infant’s health, including retinal damage. Eye protection should be used for other
patients who are close to the phototherapy device.
Warning
Patient monitoring during the phototherapy treatment
-Bilirubin level: The infant’s bilirubin level should be regularly measured during the
phototherapy treatment in accordance with the recommendations of the physician in
charge.
-Temperature: Phototherapy can affect the patient’s body temperature. The infant’s
temperature must be monitored according to the recommendations of the physician who is
responsible for the therapy. Because the phototherapy treatment may increase the body
temperature, not controlling the infant’s temperature may lead to a risk to the patient.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 4
EN
-Fluid balance: Phototherapy can affect the patient’s fluid balance. Regularly monitor the
patient’s fluid balance and take appropriate measures to maintain this balance during
phototherapy.
Note: The Bilicocoon bag system does not create sweating, however if sweating occurs it can hardly be
evacuated which can cause discomfort to the patient. To maximize the patient’s comfort in the Bilicocoon
bag system we recommend, in case of sweating, to open the device laterally on one or both sides or to
leave the patient’s arms outside of the device
Warning
Side Effects for Caregivers and People near the Device
Prolonged exposure to the phototherapy device’s blue light may cause discomfort to caregivers
such as eye irritation, nausea, headaches or dizziness. Caregivers, staff and others who are in
close proximity to the Device could be sensitive to blue light and need to protect their eyes.
Warning
Risk of Glare
Do not look at the light source (Pad Connection Port) when the Device is switched ON and the
Pad is not connected.
Hot Surface –Risk of burning
When disconnected, the Pad and Light Box connecting surfaces will be hot. There is indeed a
risk of burning (see section 5).
Warning
Cutaneous Reactions
Cutaneous eruptions such as erythema may occur in infants treated with phototherapy.
Warning
Skin Color Changes
Blue light may hinder clinical observation by masking skin color changes, such as cyanosis.
Warning
Rare Allergic Reactions
Despite the biocompatibility evaluation tests conducted in accordance with the state of the art,
rare allergic reactions may nevertheless occur.
Warning
Variation of the Ambient Conditions
Variations of the ambient conditions (room temperature and humidity, sun exposure, nearby
devices) may unfavorably affect the patient’s health, including their temperature or their fluid
balance.
Moreover, use of this device outside of the given operating conditions may compromise the
product functionality.
Warning
Reflective Foils
Never use reflective foils to increase the effectiveness of phototherapy treatment, it may
hazardously increase the body temperature.
Warning
Intravenous drugs and fluids
In order to prevent photochemical modifications, do not store drugs and infusion liquids in the
radiation area.
Warning
Combustible gases and flammable solutions
Do not use the BiliCocoon™ phototherapy system in oxygen-rich environments or in the
presence of combustible gases, such as nitrous oxide, anesthetics or any other combustible or
flammable product.
Warning
Labels Integrity and Readability
In case labels are degraded, the information addressing the safety of the patient, users, third
parties, and properties can be found in this document. Contact the supplier if information
concerning the identification of the product or of associated products is not visible anymore.
Make sure that there is no entanglement of the cord or cable at any time.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 5
EN
Warning
Symbol
Description
Symbol
Description
Manufacturer
CE Marking declaration according to
Directive 93/42/EEC
Consult instructions for use
Caution: law prohibits dispensing
without prescription
Refer to instruction
manual/booklet
Date of manufacture
Protect infants’ eyes with
opaque eye protection
Catalogue number
Type BF equipment
Serial number
The Light Box is a Class II
electro-medical device
Pad –Light Box Pairing number
Waste of electrical and
electronic equipment
Direct current
"ON" (power)
"OFF" (power)
Operating temperature
range
Hot Surface –When the Pad is
disconnected from the Light Box pay
attention to the surfaces of the two
elements that are in contact with one
another during a therapy session. This
symbol is on the product to warn the
user about the hot surfaces.
Do not spray directly over
the Connection
Do not bend the fibers at right angles
LED failure indicator
Device overheating indicator

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 6
EN
Do not store more than four
cartons on top of one
another
Maintain the carton vertically following
the arrows
Keep dry
Storage humidity range
QPS certification
Storage astmospheric pressure range
Fragile, to be handled
with care
Abbreviation
Units and Description
°C
Degrees Celsius
(Unit of temperature)
kg
Kilograms
(Unit of mass)
µW∙cm-²∙nm-1
Microwatt per square centimeter per nanometer
(Unit of spectral irradiance)
λ
Wavelength
(Unit of wavelength)
h
Hour
(Unit of time)
min
Minute
(Unit of time)
nm
Nanometer
(Unit of length)
mm
Millimeter
(Unit of length)
W
Watt
(Unit of power)
Hz
Hertz
(Unit of frequency)
VAC
Alternating current Volt
(Unit of voltage)
VDC
Direct current Volt
(Unit of voltage)
dB(A)
Decibel : Sound level –A weighting
(Unit of acoustic intensity)
hPa
Hectopascal
(Unit of atmospheric pressure)
3 COMPONENTS / SUB-ASSEMBLIES AND USER CONTROLS
3.A System Description
The BiliCocoon™ phototherapy system is composed of a blue light electronic generator –the Light Box –and
of a light-emitting fabric –the Pad –which transmits the blue light to the infant. Light Box and Pad are paired in
order to emit light with a peak between 430 and 490 nm and a spectral irradiance of 35 µW∙cm-²∙nm-1 (± 15%).
The Light Box is connected to the power grid through a specific external power module.
The BiliCocoon™ system is used with the BiliCocoon™Disposable, a non-woven disposable designed to fit the
Pad.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 7
EN
Physical Characteristics
Light Box Dimensions
215 x 198 x 160 mm3
Light Box Weight
1.4 kg
Nest Pad Dimensions
Effective light-emitting
surface of 40 ± 0.5 cm
x 30 cm
Total length 150 ± 4
cm
Bag Pad Dimensions
2 light-emitting
surfaces of 20 ± 0.5
cm x 30 cm
Total length 155 ± 4
cm
Pad Weight
< 1 kg
Optical Fibers Cable
Flexible, opaque
Approximate length 100 cm
Protection
Polyurethane enclosure which is translucent on the emitting side and opaque on
the non-emitting one
Connection
Plastic
Power Cable
3.5 ± 0.5 m
Technical Features
Mean Spectral
Irradiance
35 µW∙cm-2∙nm-1 ± 15% with disposablee. An infant would receive a lower dose if not
positioned in contact with the disposable.
The measurement is to be performed with the Ohmeda (GE) BiliBlanket Lightmeter II.
Power Supply
Input
Voltage : 100 VAC –240 VAC
Frequency : 50 Hz - 60 Hz
AC power plugs compatible with Europe and North America
Output
Voltage : 12 VDC
Current intensity : 7.5 A max
Power : 90 W max
e
This value is measured in direct contact with the disposable. The irradiance value verification should be performed without the disposable
as explained in § 4.C

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 8
EN
LEDs
Characteristics
6 LEDs of 15 W max power and emitting between 400 and 550 nm
Lifetime
The BiliCocoon™ System has an expected lifetime of at least 8000 h, corresponding to
approximately 7 years of regular use at ambient temperature.
Sound Levelf
37 dB(A) at 1 meter
Environmental Conditions
Warning
Disregarding the operating and storage conditions may lead to the Device deterioration and
failures, therefore creating risks to the patient, the users, third parties or properties.
IP21 protection for the Light Box: protection against the ingress of solid objects larger than
12.5 mm –e.g. a finger won’t have access to a hazardous part – and of dripping water –
vertically falling drops won’t have harmful effects. Although the shell provides protection
against liquid ingress, the user must take precautions to avoid the contact of the Device with
liquids, which could cause an electrical hazard.
IPX3 protection for the Pad: protection against the ingress of solids of all size and the ingress
of direct sprays of water up to 60° from vertical –in these conditions the water ingress won’t
have harmful effects. Although the enclosure provides protection against liquid and solid
ingress, the user must take precautions to prevent the infiltration of fluids, especially in the
Connection.
Do not place the system close to a source of radiant heat flow (e.g. radiant warmer). It may
impact the proper functioning of the BiliCocoon™system.
Do not place the system close to a source of moisture like a nebulizer or a steam kettle. The
moisture may affect the Light Box proper functioning and safety. Risk of electric shock.
Avoid exposure to environments with excessive dust. Lint and dust may settle in the Light
Box or on the surfaces meant to transfer blue light: the consequence would be an improper
functioning of the System and/or the deterioration of its performances.
Operating Conditions
Warning
INDOOR use only. The Device is not be used while in motion.
The Pad and the disposable can be placed in the incubator with the infant. The Light Box
must be placed outside the incubator.
Temperature range: from 10 to 35°C g
Relative humidity range: from 15 to 90%, without condensation
Atmospheric Pressure range: from 700 to 1060 hPah
Ensure that the air inlets are not covered or obstructed in order to avoid the Light Box
overheating.
The power supply to be used is the MEGMEET MANGO100-12B. The use of a different
power supply may lead to risks to the patient, users, third parties, or properties.
Storage Conditions between uses
Warning
INDOOR storage only –do not leave the system exposed to the sun. A prolonged exposure
to sun light may lead to the deterioration of the Pad optical fibers and to the system’s
performances.
Temperature range: from -25 to 50°Ci
Relative humidity range: from 10 to 90%, without condensation
Atmospheric Pressure range: from 700 to 1060 hPa
f
Value of sound pressure.
g
50 - 95 °F
h
Equivalent Altitude : 0 –3000 m (0 –9842.52 feet)
i
-13–122 °F

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 9
EN
Important
The Pad emitting surface should be stored flat, do not ever bend or stretch the emitting
surface optical fibers. Do not ever bend the optical fibers at right angles.
The cable must not be coiled more than one and a half turns. A manipulation that does not
follow the recommended instructions may damage the optical fibers and affect the Pad
lighting performances or lead to a risk to the patient's health.
Transport and Storage Conditions for Packaged Product
Warning
INDOOR storage only.
Temperature range: from -25 to 50°C j
Relative humidity range: from 10 to 90%, without condensation
Atmospheric Pressure range: from 504 to 1060 hPak
3.B Light Box Features
The BiliCocoon™ Light Box is an electronic device incorporating LEDs and emits between 430 and 490 nm.
This emission range corresponds to the absorption spectrum of bilirubin, with this light leading to bilirubine
conversion and elimination without the liver intervention.
The Light Box includes an ergonomic handle which allows an intuitive grasp.
1
Ergonomic handle
2
Front panel with display and user controls
3
Connection Port with the lenses which transfer the blue light to the Pad optical fibers
4
Side ventilation (air inlets):DO NOT OBSTRUCT
5
Back ventilation (air inlets) with dust filter (inside): DO NOT OBSTRUCT
6
Power socket (to be connected to the external power module)
7
USB Port
NOT OPERATIONAL, DO NOT USE. The symbol “CAUTION” is
close to the USB port to recall not to use this port.
Controls
Controls can be used while the user is wearing medical gloves.
j
-13 –122 °F
k
Equivalent Altitude : 0 –5500 m (0 - 18044,62 feet)

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 10
EN
1
Power switch
2
Digital Display Screen:
-Session Time (format: HH:MM, where HH stands for the hours and MM for the minutes): it’s
the time remaining before the session’s end. The colon between HH and MM flashes during
an ongoing phototherapy session.
-Total Time (format: HHHH, which stands for the hours): it’s the total time of use of the Device.
The user can obtain this information by pressing the RESET button for 10 seconds from the
system startup. This timer should not, under any circumstance, be used to evaluate the
phototherapy treatment duration.
3
+and –controls: to set session time. The session time can be set between 00:10 (= 10 min)
and 99:50 (= 99 h and 50 min). The minimum session time increment or decrement is 10 min.
4
RESET button to reset session time. The user can verify that the light indicators properly light up
by pressing this button for 10 seconds from the system startup.
5
Operation indicator: blue light strip
-Continuous light: Therapy session running
-Flashing light: Therapy session is configured but Pad is disconnected or not properly
connected.
-Light Off: A therapy session is neither ongoing nor configured.
6
Overheating indicator
-Continuous light: Fan failure
-Flashing light: Light Box overheating during treatment
7
LED failure indicator
-Continuous light: Failure of one or more LEDs
For more information on these failures, please refer to section 5.E “Failures and possible resolutions”.
3.C Pad Technical Features
The Pad is a woven fabric made of textile and optical fibers covered with a polyurethane enclosure. The Pad
Connection enables the transfer of blue light between the Pad and the Light Box.
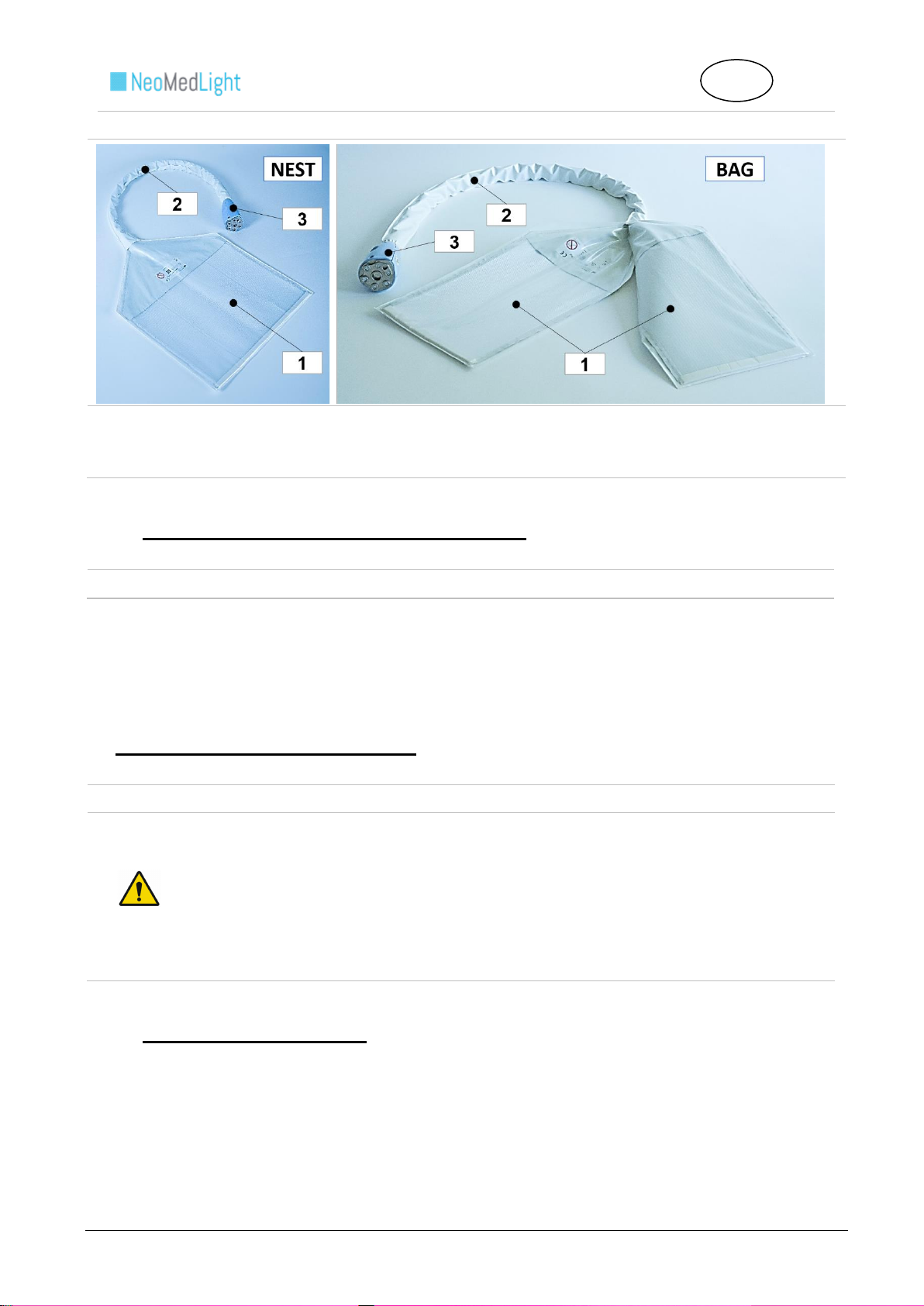
Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 11
EN
1. Lighting Surface
2. Cable
3. Connection
1. Lighting Surfaces
2. Cable
3. Connection
3.D Technical Features of the Disposable
Important
Never use the BiliCocoon™ system without the BiliCocoon™ Disposable
The BiliCocoon™ Disposable is a single-use, single-patient device, which ensures the system cleanliness
during a phototherapy treatment. (See the BiliCocoon™ Disposable Instructions for Use)
The BiliCocoon™ Bag Disposable is designed to be used with the BiliCocoon™ Bag Pad.
The BiliCocoon™ Nest Disposable is designed to be used with the BiliCocoon™ Nest Pad.
4 INSTALLATION AND USE
Important
Beforehand, read this manual, paying particular attention to its warnings.
Warning
The phototherapy system may increase the patient’s body temperature when it’s used in
combination with a thermotherapy system (e.g. infant incubators, infant transport incubators,
infant radiant warmers, devices supplying heat via blankets, pads or mattresses). In this case
the user should measure the infant’s body temperature directly (i.e. on the skin’s surface)
and use the baby controlled mode of these devices, otherwise the set air temperature of the
incubator or the heater output of the radiant warmer or heated mattress has to be adjusted
according to the body temperature measurements.
Place the Light Box outside of these systems.
4.A Light Box Positioning
The BiliCocoon™ should only be used according to the described operating conditions (see section 3.B
Light Box Features).
If the Light Box was stored under environmental conditions outside of the operating condition range, place
the Device in the proper operating conditions for at least 1 hour before using it and let its temperature
stabilize.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 12
EN
1. Verification
•Verify that the Light Box is not damaged
•Check before each use that the power cord and the power supply do not show any abnormality.
Warning
Risk of Electric Shock
Disregarding the verification instructions may inhibit the detection of a system malfunction or
failure and lead to risks to the patient’s health or to the user.
2. Positioning:
•Place the Light Box on a flat, stable surface
•Take care not to block the side (left as in the picture and right) and rear air
inlets.
•Make sure not to position the Device or the external power supply so that
the power cord would be difficult to unplug in case of emergency.
•Do not use adjacent to or stacked with other equipment.
•Consider not to place the infant too close (< 30 cml) to the Light Box.
Warning
If adjacent or stacked use is necessary, the Light Box should be observed to verify normal
operation in the configuration in which it will be used.
3. Connection of the Power Supply to the Light Box
•Connect the Power Supply in the power socket
4.B Pad Setup
1. Verification and Cleaning
•Make sure the Pad enclosure does not present sharp edges which may injure the infant.
•Make sure the Connection is not broken
•Clean the Pad before and after each use (see Chapter 5.C Pad Cleaning)
•Check the number to the right of the "PAIR" symbol on both the Pad and on the Light Box labels:
these numbers must match.
l
≈11.8 inches

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 13
EN
Warning
If the following defects are detected, discontinue use of the Pad:
-Cracks or holes in the polyurethane enclosure
-Cracked or broken Connection
-Presence of sharp edges
Warning
Do not cut or scratch the Pad
Take care to avoid dropping or handling the Device in a way that may cause the Connection
to deform.
2. Positioning
•Place the Pad on a flat, stable surface
3. Connection of the Pad with the Light Box
•Connect the Pad with the Light Box by inserting the
Pad Connection (1) into the Connection Port (2).
•Make sure the Pad and the Light Box are well
connected.
•Make sure the cable and the light-emitting area are
not bent or stretched.
Warning
Use or manipulation of the Pad outside of these recommendations may damage the optical
fibers and affect the Pad lighting performances or lead to a risk to the patient's health.
4.C Verification of the Irradiance Value
Warning
Prior to insertion of the Pad in the disposable, measure the irradiance value on the
Pad lighting surface.
If this step is neglected, there is a risk of under-exposure –leading to an ineffective
treatment –or a risk of over-exposure.
Recommended Radiometer: BiliBlanket Lightmeter II from Ohmeda (General Electrics). For more
information on how to use the radiometer refer to its instructions for use.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 14
EN
The blue light emission is stable from the session start: irradiance measurements can be taken as soon as
the phototherapy session is active.
1. Remove the Receptor Cap (1)
2. Switch On the Radiometer using the ON/OFF switch (2) on the side of the device as in the picture. Check
that the Hold-Run (3) is released (i.e. the display reading is not frozen). In case the display is frozen push
the Hold-Run to release it.
3. Measure the blue light from the environment while the
Device is turned off. Place the radiometer with the Light
receptor dome (4) oriented upward as in the picture on the
right. The displayed value should be lower than 1 μW∙cm-
2∙nm-1.
4. Connect the Pad to the Light Box, switch the Device on and launch a test session. The Pad now emits
blue light. See section 4.E.
5. To make a measurement place the light receptor dome (4)
in contact with the surface as in the picture –in this
position the user looks at the back of the radiometer.
6. Place the light receptor dome on the measurement point
n° 1 as in the picture
7. Leave the light receptor dome in this position for few
seconds.
8. Press the Hold-Run (3) which freezes the measurement displayed on the screen.
9. Turn the radiometer, read and note the value displayed on the screen (5).
10. Push the Hold-Run (3) again to unfreeze the displayed measure.
11. Repeat steps 5 to 10 for the 7 other measuring points as shown in the pictures.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 15
EN
For the Nest Pad
Consider the 8 measuring points as identified below
For the Bag Pad
Consider the 4 measuring points on each
small Pad as identified below (8 measuring
points totally)
12. Calculate the mean value over the eight measures. This value should be in the range defined in the table
below.
Average spectral irradiance (I - µW∙cm-²∙nm-1)
over the eight points
Conclusion
38 < I < 43m
Optimal Irradiancen
I < 38 or I > 43
Out of specification –refer to Section 5.F
“Maintenance Operations”
Important
Refer to section 5.E in case the irradiance value is outside of the defined range and inform
the manufacturer.
Important
The use of a BiliCocoon™ system with a mean irradiance value exceeding the defined range
upper limit, may deteriorate the Pad’s optical fibers.
4.D Setup of the Disposable and Positioning of the Infant
Warning
For any information on the Disposable and its use, please refer to the last version of
the Instructions for Use of the BiliCocoon™ Disposable.
The BiliCocoon™ Disposable is not intended to be used with a phototherapy
treatment system other than the BiliCocoon™.
Warning
The BiliCocoon™ Disposable is a single use, single-patient medical device. It should be
used respecting the usual hygiene precautions. There is indeed a cross-contamination risk
if the Disposable is used for more than one patient.
Never place the infant directly on the Pad. Always use the Disposable specifically designed
for the BiliCocoonTM.
The Disposable is not designed for re-use and should not be cleaned, disinfected, or placed
in contact with the cleaning products used to clean the BiliCocoon™ Pad.
Not using the disposable or having the cleaning products in contact with the infant may lead
to allergic reactions for the patient.
m
The user should refer to the radiometer instructions for use and take into account its accuracy (±3%).
n
Defined according to: “Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation“, Pediatrics
2004; 114:1 297-316; doi:10.1542/peds.114.1.297, prepared by the AMERICAN ACADEMY OF PEDIATRICS.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 16
EN
Warning
Infants should wear a diaper during a phototherapy session.
Warning
Do not place the infant too close (< 30 cmo) to the Light Box.
Warning
Check that the packaging of the BiliCocoon™ Disposable is not damaged: The use of a
device whose cleanliness is intended as a main feature and controlled thereof, but whose
packaging is damaged, may lead to a risk to patient's health.
Warning
Ensure that the Pad is clean and dry. The BiliCocoon™ Disposable should not come into
contact with the cleaning and disinfectant agents used to clean the Pad.
Important
Select the Disposable according to the type of Pad (Nest Disposable or Bag Disposable)
and install the Disposable according to the Disposable Instructions for Use.
Important
We recommend not to use the Bag System for babies weighing less than 2500g
Warning
Ensure that the Pad is inserted right to the end of the BiliCocoon™ Disposable pocket and
that the Disposable pocket hasn’t been damaged by this insertion.
Warning
Place the infant on the Disposable with the baby’s legs towards the Pad cable. Never place
the infant's head close to the Pad cable. This positioning may otherwise lead to a risk of
strangulation for the patient.
Important
Ensure that the maximum of the skin surface area will be in contact with the light-emitting
area.
Warning
Once the infant has been properly installed protect the infant’s eyes with suitable eye
protection.
The infant in the
BiliCocoon™ Nest
System: the Nest Pad is
inserted in the Nest
Disposable; the infant is
then placed on the
disposable in the zonep
defined by the red line as
in the picture.
The infant in the
BiliCocoon™ Bag
System: the Bag Pad is
inserted in the Bag
Disposable; the infant is
then placed on one of the
internal faces of the
disposable in the zoneq
defined by the red line as
o
≈11.8 inches
p
The zone defined by the dashed black line represents the part of the Device the infant is in contact with. Please refer to the
Instructions for Use of the BiliCocoon™ Disposable for more details.
q
The zone defined by the dashed black line represents the part of the Device the infant is in contact with. Please refer to the
Instructions for Use of the BiliCocoon™ Disposable for more details.

Instructions for Use :
BiliCocoon™ Phototherapy System
IFU-BCC-SY-EnFr-2017-10-v13 17
EN
in the picture; the other
surface is folded on the
infant’s chest.
XXX
4.E Performing a Treatment
Precautions
Warning
Before beginning the treatment check the installation and the Light Box / Pad connection.
When the Device is switched ON but the Pad is not connected, do not look at the Pad
Connection port.
Warning
During the session or at its end, the temperature of the Connection surface which is in contact
with the Light Box can be high. There is a risk of burning –see section 5. Take care in
handling the Device.
Warning
Keep the Light Box steady with one hand when disconnecting the Pad through its
Connection. Pulling the Connection without keeping the Light Box may lead the Light Box to
fall, be damaged and harm users and other people.
Setting a Session
1. Turn ON the Light Box using the ON/OFF black
switch (1)
2. Set the session time (HH:MM) using the +and –
controls (2). If a session was previously set refer
to the following section.
3. The session automatically begins in 5 seconds
with the Device emitting blue light through the disposable. Check that the Pad and the Light Box are well
connected: the Blue line feature light (3) should be continuous. In case the light flashes or is off, refer to the
table "Failures and possible resolutions".
4. When the session starts all the controls become inactive. To reactivate them, switch OFF and ON the
Device.
5. The session will stop automatically when the session time will be 00:00.
Resetting a Session or Changing the Settings of a Session
1. Switch OFF (1)
2. Switch ON (1)
3. To set the session time to zero press the RESET (4) button within 5 seconds from the switch ON
4. Set the session time (HH:MM) using the +and –controls (2) within 5 seconds after powering up.
Interrupting a Session
1. Switch OFF (1) to interrupt the session.
2. Switch ON (1) to restart a session: The system displays the last session time.
This manual suits for next models
1
Table of contents
Languages:
Other NeoMedLight Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual













