This confidential document
is the property of Aktiia
P01_0020 - USER MANUAL Version: 2
Printed copies are uncontrolled - Check you are using the latest version Page 4 of 14
Introduction
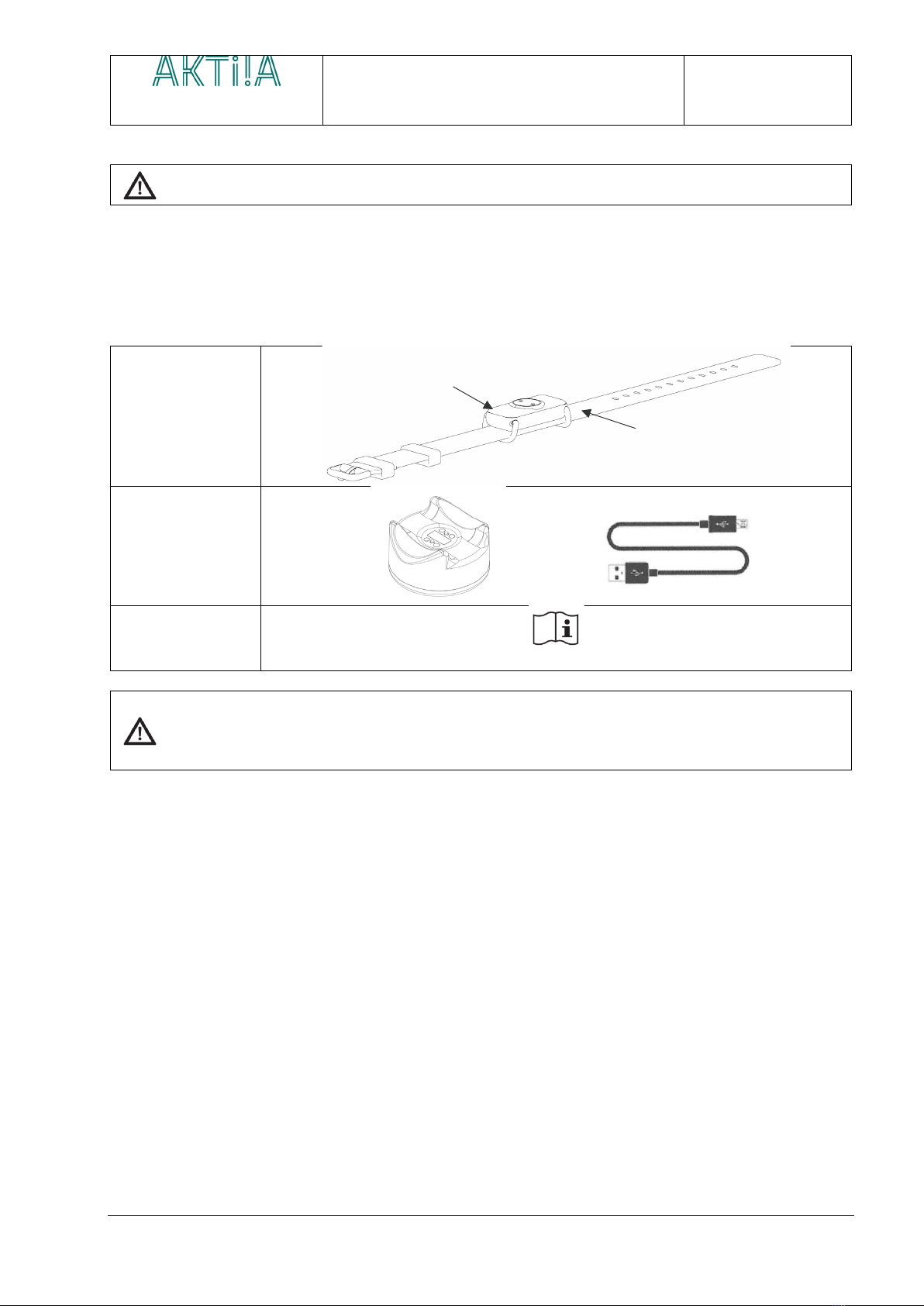
AKTIIA bracelet is the smart way to track your systolic and diastolic Blood Pressure and Heart Rate over days and
nights without any effort. AKTIIA bracelet is designed to be discreet, with no lights or alarms bothering you during
your daily tasks. With its ease of use and accuracy, AKTIIA is the perfect solution to track your blood pressure 24/7.
AKTIIA offers a new way for monitoring your heart health that easily integrates into your life. Our device tracks
your Blood Pressure through unnoticeable measurements performed multiple times per day. All you need to do
is to wear the bracelet. You can access your data anytime by simply looking at the AKTIIA smartphone app.
www.aktiia.com/ifu
Please read carefully this User Manual to gain a complete understanding of the device’s
functions and safety-related information. In case you have any additional questions, you
encounter any issue, or you would like to suggest some improvements, please contact AK-
TIIA’s Customer Service at support@aktiia.com or visit our website at www.aktiia.com
1. Intended Use and technological characteristic
AKTIIA bracelet is intended for non-invasive intermittent blood-pressure (BP) monitoring in adult population.
AKTIIA bracelet is intended to be used 24/7 in everyday life conditions. However, AKTIIA bracelet will provide
blood pressure measurement only when the user is motionless.
AKTIIA Bracelet uses a PPG technology (Photoplethysmography) to acquire reflective optical data on your wrist,
using a green LED. The PPG data are then transferred through the AKTIIA App to a secured cloud server on which
a PWA (Pulse Wave Analysis) algorithm estimate your blood pressure from the optical data.
2. Contraindications
AKTIIA Bracelet IS NOT INTENDED to be used in case of tachycardia (heart rate at rest > 120bpm), atrial
fibrillation, diabetes, renal dysfunctions (eGFR < 60mL/min/1.73 m2), hyper-/hypothyroidism, pheo-
chromocytoma, pregnancy, arteriovenous fistula.
AKTIIA Bracelet should not be applied on damaged / injured skin.
AKTIIA Bracelet is not intended for users below 21 y.o. and above 65 y.o.
3. Cautions
AKTIIA monitor is not intended to be a diagnostic device. Self-diagnosis of measurement results and
self-treatment are potentially dangerous. You should always consult your doctor for relevant interpre-
tation of blood pressure results.
This device may only be used for the purposes described in this User Manual. Aktiia cannot be held
liable for damage or injury caused by incorrect use. Always follow the operating procedures described
in this User Manual to measure your blood pressure accurately and safely.
AKTIIA bracelet is designed as a device for personal use (single user) only. Do not share your device
with others as it may result in inaccurate blood pressure readings.