B.O.L.T BT4 User manual

ONE-TOUCH WIRELESS HEALTH TRACKER
BODY LIFE TRACKER
B.O.L.T User Manual
Product Names: B.O.L.T BT4
Device Model Numbers: VA01, VA01-0, VA01-1, VA01-2
Document No : AMZ-PRD-UM-B-BT4
Version : 1.2
Date : 26.08.2019

B.O.L.T User Manual
2
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Legal
Disclaimer
This publication/content contains proprietary information which is protected by copyrights. No
part of this publication may be reproduced, transcribed, stored in a retrieval system, translated
into any language or computer language, or transmitted in any form whatsoever without the
prior written consent of the publisher. AmZetta Technologies Private Ltd. retains the right to
update, change or modify this publication at any time, without notice.
For Additional Information
Call AmZetta Technologies Private Ltd at 1800-572-4061.
Limitations of Liability
In no event shall AmZetta Technologies Private Ltd be held liable for any loss, expenses, or
damage of any kind whatsoever, whether direct, indirect, incidental, or consequential, arising
from the design or use of this product or the support materials provided with the product.
Limited Warranty
No warranties are made, either expressed or implied, with regard to the contents of this work,
its merchantability, or fitness for a particular use. AmZetta Technologies Private Ltd assumes no
responsibility for errors and omissions or for the uses made of the material contained herein or
reader decisions based on such use.
Trademark and Copyright Acknowledgments
Copyright © 2019 AmZetta Technologies Private Ltd. All Rights Reserved.
AmZetta Technologies Private Ltd.,
Kumaran Nagar, Semmenchery, Off Rajiv Gandhi Salai (OMR)
Chennai (TN) 600119, India.
All product names used in this publication are for identification purposes only and are
trademarks of their respective companies.

B.O.L.T User Manual
3
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Document History
Version
Date
Comment
1.0
15-Jul-2019
Initial Release
1.1
23-Aug-2019
Bluetooth security labelling
1.2
26-Aug-2019
Spo2 Pulse rate range updated

B.O.L.T User Manual
4
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Table of Contents
1. Intended Use ...................................................................................... 6
2. Product Description ............................................................................. 7
3. Know Your Unit................................................................................... 9
4. Package Contents of B.O.L.T .................................................................10
5. Product Quality, Reliability and Safety .....................................................10
5.1 Essential Performance ....................................................................11
5.2 Warning......................................................................................11
5.3 Caution .....................................................................................14
5.4 Care and Maintenance ....................................................................16
5.5 Calibration Verification Procedure.....................................................17
5.6 Disposal ......................................................................................17
6. Contraindications ...............................................................................17
7. Product Features................................................................................17
8. Operating Environment ........................................................................18
9. Prerequisites.....................................................................................18
10. Using B.O.L.T Application...................................................................18
10.1 Downloading and Installing B.O.L.T application on your mobile device ........18
10.2 Using Sliding and Settings Screens...................................................21
11. Blood Pressure Measurement Procedure ................................................22
11.1 Blood Pressure measurement with B.O.L.T........................................23
11.2 Blood Pressure Classification for Adults ............................................25
12. Blood Oxygen Level Measurement Procedures using Pulse Oximeter..............26
12.1 Blood Oxygen Level measurement with B.O.L.T..................................26
12.2 Blood Oxygen Level Classification ...................................................28
13. Temperature Measurement Procedures using Infrared Thermometer.............29
13.1 Body Temperature measurement with B.O.L.T ...................................30
13.2 Temperature Classification ...........................................................31
14. Body Mass Index (BMI) .......................................................................31
14.1 Measuring BMI with B.O.L.T...........................................................31
14.2 BMI Classification........................................................................31
15. Battery Care...................................................................................32
15.1 Replacement of Battery Pack.........................................................33
16. Troubleshooting ..............................................................................34
17. Explanation of Indicators/Symbols ........................................................37
18. Acronyms Used................................................................................39

B.O.L.T User Manual
5
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
19. Technical Specifications ....................................................................40
20. Certifications..................................................................................44
21. Emissions and immunity information .....................................................47
21.1 Electromagnetic emissions ............................................................47
21.2 Electromagnetic Immunity ............................................................48
22. Warranty Information........................................................................50
23. Customer Care Support ..................................................................... 50
24. Contact us ..................................................................................... 51

B.O.L.T User Manual
6
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
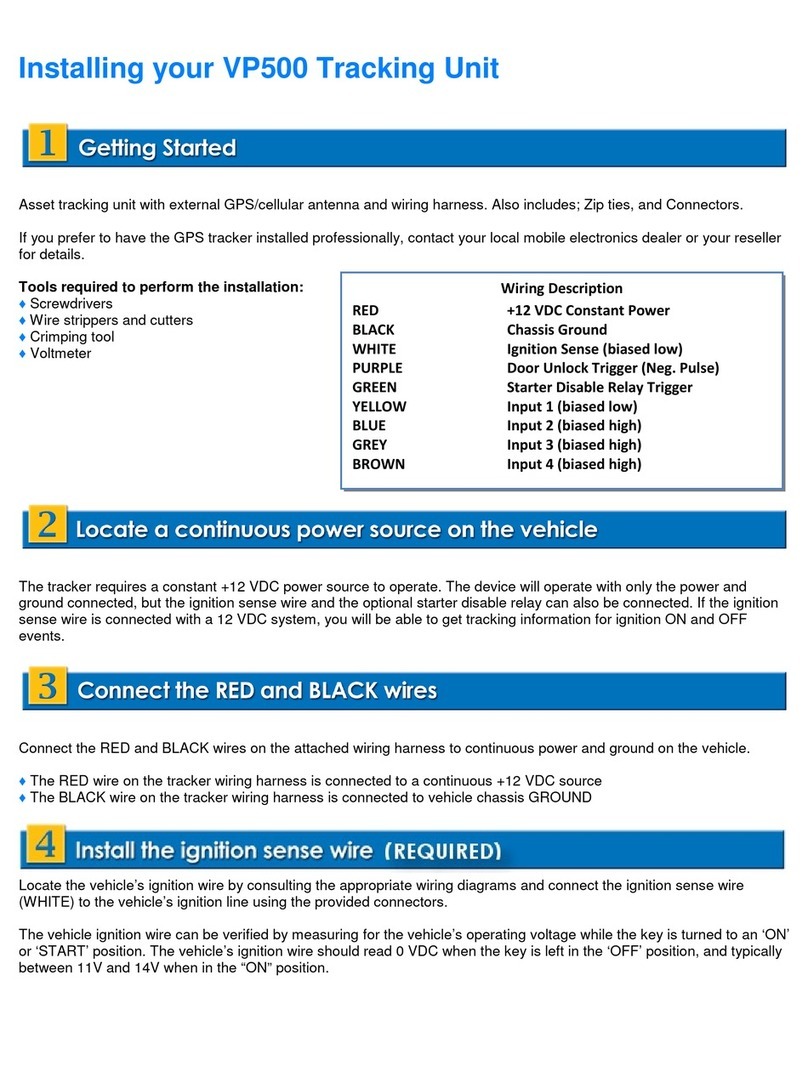
NIBP Monitor (Systole, Diastole and Pulse Rate), Infrared Thermometer
(Temperature), Pulse Oximeter (SpO2 & Pulse Rate)
1. Intended Use
Product Name: B.O.L.T BT4
Device Model: VA01, VA01-0, VA01-1, VA01-2
B.O.L.T model (VA01) Base unit with it`s accessory devices attachment (VA01-0, VA01-1 and
VA01-2) and the B.O.L.T application is a patient vitals measuring and monitoring system used
for spot-checking and measuring the physiological parameters of adult patients / users (18
years of age or older). The physiological parameters measured and monitored are:
Non-Invasive Blood Pressure (NIBP), Pulse Rate (PR)
Oxygen saturation (SpO2), Pulse Rate (PR)
Body Temperature (TEMP)
B.O.L.T application is downloaded and installed in a Bluetooth enabled smart devices/
computer. The operations of the base unit and the attached devices like start and stop are
controlled by the B.O.L.T application and the results are displayed by the B.O.L.T application
on the screen of the smart devices/ computer.
This device is applicable for use by an adult and it can be used in a clinical setting like a
physician office and also in a home environment for patients to keep track of the
physiological parameters mentioned above. Home users are advised to contact their
physician if any abnormal values are indicated.
B.O.L.T, NIBP model Base unit (VA01) along with an inflatable cuff (VA01-0) that is wrapped
around the upper arm, is a fully automatic, non-invasive, wireless blood pressure
measurement system intended to measure the diastolic and systolic blood pressures and
pulse rate of an adult individual.
B.O.L.T, IRT model Base unit (VA01) along with a thermometer probe (VA01-1) using infrared
sensor detects body temperature from the ear canal in the adult population.
B.O.L.T, Pulse Oximeter model Base unit (VA01) along with a pulse oximeter probe (VA01-2) is
a non-invasive device intended for spot checking of functional oxygen saturation of arterial
hemoglobin (SpO2) and pulse rate (PR) from the fingertip of adult users.

B.O.L.T User Manual
7
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
2. Product Description
The B.O.L.T Patient Physiological Monitor which is called as B.O.L.T Life tracker is a patient
vitals measuring and monitoring device which can be used by home users as well at a clinical
setting like physician office for spot-checking and measuring of the following patient’s
physiological parameters:
Measurement of the Systolic Blood Pressure, Diastolic Blood Pressure and Rate (PR),
non- invasively;
Spot check of functional blood Oxygen saturation (SpO2) and Pulse Rate (PR), non-
invasively; and
Measurement of the Body Temperature through auditory canal, non invasively.
The B.O.L.T system is modularly designed and consists of:
oBase unit (model VA01);
oNIBP (Non-Invasive Blood Pressure) Cuff (model VA01-0);
oInfrared Radiation Energy Technology-IRT Thermometer (model VA01-1); and
oPulse Oximeter (VA01-2).
Only the IRT Thermometer or Pulse Oximeter device can be connected at a time to the base
unit. The NIBP cuff can be connected permanently to the Base unit. The system is packaged
as a complete system with all the devices included or alternatively packaged separately with
the base unit and individual devices based on the model chosen by the user.

B.O.L.T User Manual
8
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Base Unit (Model VA01)
The B.O.L.T patient monitor system is elegantly designed with a small form factor. The device
has two LED indicators that display power status and Bluetooth connectivity status. The
device has an on/off slide switch on the side and a USB power socket. The device has one
accessories port with an accessory connector to connect IRT Thermometer and Pulse
Oximeter. There are two other ports to connect the twin tubes of the NIBP Cuff. The base
unit has a rechargeable lithium polymer battery and the device should be used only in battery
mode and it should not be used while charging. The power adapter is given only for charging
the device.
The system is controlled and operated via the B.O.L.T application which is downloaded and
installed in a Bluetooth enabled smartphone/tablet/computer. The B.O.L.T application is
supported for the following versions of Android/iOS.
The Android OS version 4.0 or higher.
The iOS version 9 or higher.
Windows SDK is also available for the Windows desktop users which
will be provided on request.
The software communicates with the base unit (VA01) via Bluetooth communication to the
connected smartphone/tablet/computer. The user has to pair the base unit with handheld
mobile devices or computers through Bluetooth and utilizes the Software application to
operate and control the devices attached to the base unit. Functions like activating and
stopping the device and display of the test values are performed by the B.O.L.T application
residing in the Bluetooth connected smartphone/tablet/computer. Either the SpO2 probe or
IRT probe is connected to the accessory port of the base unit for the measurement. The base
unit takes signals from the connected device like NIBP Cuff, SpO2 probe, IRT Thermometer.
The software and firmware of the B.O.L.T patient monitoring system, process the data from
the accessory devices, then displays the parameters/measured data on the screen of a
wirelessly connected Bluetooth device like a smartphone/ tablet/ personal computer. B.O.L.T
application can hold any number of records and recognized by user ID. The number of
records is limited by the data storage capacity of the secured cloud infrastructure.
The data collected from devices can be securely uploaded/stored in a secured cloud. The
secured cloud is HIPAA compliant and hosted in a HIPAA compliant data Centre. All the
protected health information and the physiological data of a user/patient is encrypted at rest.
The communication over the internet to the cloud utilizes the HTTPS/TLS protocol. The
B.O.L.T is also packaged with an SDK (Software Development Kit) to integrate the reading
from the devices to an external system like a Care Portal/Electronic Medical Record software
system.

B.O.L.T User Manual
9
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Non-Invasive Blood Pressure (Model VA01-0)
NIBP Cuff (model VA01-0) measures non-invasively the blood pressure (the pressures of
systolic and diastolic) and pulse rate (PR). The NIBP is based on the oscillometric method. The
NIBP is connected to the base unit via the accessory port in the base unit.
Pulse Oximeter (Model VA01-2)
The pulse oximeter (model VA01-2) measures the patient’s blood oxygen saturation (SpO2)
and pulse rate (PR) non-invasively on a finger. The pulse oximeter is based on the
photoelectric method. The Pulse oximeter is connected to the base unit via the accessory
port in the base unit.
IRT Thermometer (Model VA01-1)
The IRT thermometer (VA01-1) measures body temperature in the ear canal. The IRT
thermometer is based on infrared radiation energy technology (IRT). The IRT thermometer is
connected to the base unit via the accessory port in the base unit.
3. Know Your Unit
The base unit has an elegant look and feel with two LED indicators, BP Sockets for connecting
the BP Cuff connector plug, an accessory connector (Accessory connector), a micro USB
connector for Charging the device, and a power ON/OFF sliding switch as shown below.
Accessories like a cuff, IR Thermometer with Probe cover, SpO2 probe, and USB cable with
power adapter are provided along with the base unit.

B.O.L.T User Manual
10
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
4. Package Contents of B.O.L.T
S. No
Packing List
Quantity
1
Base Unit (VA01)
1
2
Cuff (VA01-0)
1
3
IR Thermometer (IRT) Probe
(VA01-1)
1
4
Probe Cover
1 pack
5
SpO2 probe (VA01-2)
1
6
Power Adapter
1
7
USB Cable
1
8
Warranty Card
1
9
Quick Reference Guide
1
10
Base Unit USB Plug
1
11
Accessory Connector Plug
1
12
Travel Bag
1
5. Product Quality, Reliability and Safety
The B.O.L.T devices have been designed with an emphasis on quality, reliability and safety,
but the manufacturer can only accept responsibility for these aspects, provided the following
conditions are met.
The B.O.L.T devices should be used as per the operating instructions mentioned in the
user manual provided by the manufacturer.
All modifications and repairs to the devices should be carried out by authorized
manufacturer personnel or their agents.

B.O.L.T User Manual
11
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
The user must follow the regulations specified in the below warnings, cautions and
Care & maintenance.
5.1 Essential Performance
The ability to detect noninvasive blood pressure (NIBP) as per IEC 80601-2-30, noninvasive
monitoring of functional oxygen saturation of arterial hemoglobin (SpO2) as per ISO 80601-
2-61, and body temperature (TEMP) as per ISO 80601-2-56 & ASTM E1965-98 (2016) .
5.2 Warning
All users should read the user manual thoroughly. An electronic version of the current
copy of this manual can be obtained from the official website.
Read the Intended use, Warnings and Cautions carefully before the use of the
product.
All modifications and repairs to the B.O.L.T devices should be carried out by
authorized service personnel or their agents.
Do not plug or unplug the AC adapter with wet hands.
Do not clean the device while the power is ON.
Use of the devices are restricted to one patient at a time.
Highly recommended to use manufacturer supplied power adapter and USB cable for
charging the B.O.L.T base unit.
Keep the B.O.L.T devices out of reach of infants, children, or pets, since inhalation or
swallowing of small parts is dangerous or even fatal.
If you encounter any problem while taking blood pressure measurement, such as
inflation does not stop or air leakage in the cuff, remove the cuff or pull out the air
tube from the base unit.
Do not wrap the cuff around the arm where an intravenous injection or transfusion is
being given to the patient.
Do not operate the B.O.L.T device in places where inflammable gas, such as highly
inflammable anesthetic, may be generated or in a high-pressure oxygen room or in an
oxygen tent.
Keep the B.O.L.T device away from infants, small children or anyone who is incapable
of using the device in a correct manner.
Do not use the device for a cardio-pulmonary bypass patient.
Do not use the device in an MRI suite.
The B.O.L.T device is not suitable for use on pregnant women, patients with an
implanted electrical device, patients with preeclampsia, premature ventricular beats,
atrial fibrillation, peripheral arterial disease, and patients undergoing intravascular

B.O.L.T User Manual
12
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
therapy or arterio-venous shunt or people who received a mastectomy. Consult your
doctor prior to using the device if you suffer from any of these illnesses or conditions.
Retake the reading when the application displays unexpected errors/readings by
following the mandatory instructions given in the corresponding measurement
sections.
Regularly check that the operation of the B.O.L.T device does not result in the
prolonged impairment of patient blood circulation.
Do not use the B.O.L.T device to monitor the user who is operated with HF surgical
equipment.
Clean the device and accessories with a soft cloth dampened with 70% isopropyl
alcohol before taking a measurement from one user to another to avoid cross-
contamination.
The B.O.L.T device should not be used adjacent to or stacked with other equipment,
and that if adjacent or stacked use is necessary, the device should be observed to
verify normal operation in the configuration in which it will be used.
Pressurization of the cuff due to simultaneous use of the cuff on the same limb can
temporarily cause loss of the function.
The B.O.L.T device is designed for adults and not recommended for the neonates,
pediatrics, and infants.
The user is recommended to insert the given USB plug in the USB port of the base
unit during battery mode operation.
Inserting USB Plug Zoomed View
Removing USB Plug Zoomed View
The user can remove the USB Plug while charging the device.

B.O.L.T User Manual
13
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
The user is recommended to insert the given accessory connector plug in the
accessory connector port when the accessory is not connected.
Inserting Accessory Connector Plug Removing Accessory Connector Plug
The user can remove the Accessory connector plug while connecting the accessory.
Keep the USB plug and Accessory connector plug safe and use as per the above
instructions to protect the device from dust and to meet the ESD requirements.
Please do not use the same cuff for patients who are highly infectious as this practice
may cause cross-infection.
Avoid using cuff over a wound area as this may cause further injury.
Do not use the cuff other than arm area.
Do not kink the connection tube during the measurement. The cuff pressure might
continuously increase which could prevent the blood flow and result in injury.
Use probe cover for the IR Thermometer probe during the temperature
measurement.
If the thermometer probe is ever accidentally used without a probe cover attached
the readings may be incorrect. In that case, clean the lens with a soft cloth or cotton
swab moistened with alcohol.
This thermometer probe (VA01-1) must only be used with manufacturer
provided/recommended Soft Touch (PC 20) probe covers.
Place the finger inside the SpO2 probe properly during SpO2 measurement.
Disposable like Probe cover is meant for single user / Single use only. The probe cover
needs to be disposed after the single use to avoid cross- contamination.
While on the finger, do not press the pulse oximeter device against any surface and
do not squeeze or hold it together. The internal spring provides the correct pressure;
additional pressure may cause inaccurate readings.
Inspect the sensor application site at least every 6 to 8 hours to ensure correct sensor
alignment and skin integrity. Patient sensitivity to sensor may vary due to medical
status or skin condition.
The misapplication of the SpO2 probe with excessive pressure for extended periods of
time may cause a pressure injury to the patient.

B.O.L.T User Manual
14
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
The responsible organization and the user must verify the compatibility of the display
and SpO2 probe before use to avoid patient injury.
This probe is only for use with the B.O.L.T device. Do not attempt to use this probe
with other medical devices.
It is not recommended to operate the SpO2 probe greater than 41°C.
No components made with natural rubber latex are used in the components that
come in patient tissue contact.
The B.O.L.T device has been designed to pair with a single tablet/mobile phone during
patient monitoring activities. It is not possible to view patient data from multiple
tablets/mobile phones.
Exposure to unsuitable environmental conditions may cause the device to
malfunction and provide inaccurate readings.
If the user is uncertain about the accuracy of any measurement, it is the responsibility
of the user to verify the vital signs by an alternative means if deemed necessary and
make certain that the accessories and the device are functioning properly.
Use of accessories, transducers, or cables with the B.O.L.T device other than those
specified may result in increased emissions or decreased immunity of the B.O.L.T
device.
Portable RF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 30 cm (12 inches) to any part of
the B.O.L.T device including cables specified by the manufacturer. Otherwise,
degradation of the performance of this device may occur.
To avoid measurement errors, avoid taking measurements near a strong
electromagnetic field radiated interference signal.
Highly recommended for changing the battery of the base unit at the authorized
service center.
5.3 Caution
Do not use the device while moving in a vehicle as this may result in erroneous
readings.
Do not use the device in the Intensive and critical care unit in the hospital
environment.
Do not use any other branded cuffs as this may result in inaccurate measurements.
Do not use unauthorized parts and accessories for the measurement which may
result in inaccurate readings.
Flashing of the red power LED indicates the critically low level of the battery.
Do not store or use this device in locations which are maintained above the specified
temperature and humidity ranges.

B.O.L.T User Manual
15
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Do not expose the thermometer to the temperature extremes below 14 °F or over
131 °F (- 10 / 55 °C) or excessive humidity (> 85 % RH non-condensing).
Nail polish or false fingernails may cause inaccurate SpO2 readings.
SpO2 measurements may be adversely affected in the presence of high ambient light,
physical movement, low perfusion, dysfunctional hemoglobin, presence of certain
dyes, etc.
The device may not work when circulation is reduced. Warm or rub the finger, or re-
position the device.
Pulse Oximeter (SpO2 probe) device has no audible alarms and is intended only for
spot-checking.
Pulse Oximeter (SpO2 probe) is designed to determine the percentage of arterial
oxygen saturation of functional hemoglobin. Factors that may degrade pulse oximeter
performance or affect the accuracy of the measurement include the following:
•do not apply the pulse oximeter on the same arm as a blood pressure cuff,
arterial catheter or infusion line(s) (IVs)
•excessive light, such as sunlight or direct home lighting
•excessive motion
•moisture in the device
•improperly applied device
•finger is outside recommended size range
•poor pulse quality
•venous pulsations
•anemia or low hemoglobin concentrations
•cardio-green and other intravascular dyes
•carboxyhemoglobin
•methemoglobin
•dysfunctional hemoglobin
•artificial nails or fingernail polish
•residue (e.g., dried blood, dirt, grease, oil) in the light path
Using the tablet/mobile phone in high ambient light conditions may affect the ability
to read the values correctly.
Use only manufacturer-specified batteries and charging devices.
Do not sterilize, autoclave, or immerse this device in liquid. Do not pour or spray any
liquids into the device.
Portable and mobile RF communications equipment including CT, diathermy, RFID,
and electronic article security systems can affect medical electrical equipment.
This equipment complies with IEC 60601-1-2 for electromagnetic compatibility for
medical electrical equipment and/or systems. This standard is designed to provide
reasonable protection against harmful interference in a typical medical installation.
However, because of the proliferation of radio-frequency transmitting equipment and
other sources of electrical noise in health care and other environments, it is possible
that high levels of such interference due to close proximity or strength of a source
might disrupt the performance of this device. Medical electrical equipment needs

B.O.L.T User Manual
16
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
special precautions regarding EMC, and all equipment must be installed and put into
service according to the EMC information specified in this manual.
5.4 Care and Maintenance
Store the device and the accessories in a clean and safe location.
User is highly recommended to follow the instructions provided in this user manual
for safe operation.
Changes or modifications not approved by the manufacturer will void the user
warranty.
Keep the accessories and device away from rain, sweat, and water.
Avoid operating the B.O.L.T device in dusty and unstable temperature environments.
Use the B.O.L.T device in the environment as described in this user manual.
Otherwise, you will compromise the device’s performance and reduce its lifetime.
Do not disassemble, repair, or modify the device by yourself, as this could affect the
safety, and voids the warranty of the device.
The B.O.L.T device and accessories are non-sterile. Use a soft cloth to clean the
B.O.L.T device. Do not use any abrasive or volatile cleaners. If necessary, you may
wipe the cuff with a soft, damp cloth.
Clean the rubber touching the finger inside the SpO2 probe gently by a soft cloth
dampened with 70% isopropyl alcohol before measurement.
Clean the IRT sensor window with a soft cloth dampened with 70% isopropyl alcohol
before measurement.
Unexpected mechanical damage of the device must be intimated to the manufacturer
immediately.
Do not service or perform maintenance procedure while in use with patients.
Servicing and troubleshooting procedures on the software or hardware components
may only be performed by authorized service centers.
Any firmware/software update to B.O.L.T device has to be done through an
authorized service center of Amzetta Technologies Pvt Ltd. The B.O.L.T application will
prompt the user, when updates are required.
For service or consumables contact Toll-free number (India): 1800–572–4061, (US)
1833-222-5444 or email to [email protected]
The service life of the B.O.L.T device, IRT & SpO2 are 5 years.
The service life of the Cuff & dust cover are 3 years.

B.O.L.T User Manual
17
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
5.5 Calibration Verification Procedure
This calibration verification procedure, technical descriptions, circuit diagrams, component
part lists and assembly instructions are restricted to access by the responsible service
personnel and is not applicable in normal usage.
5.6 Disposal
Dispose the device, accessories, and consumables according to local disposal and recycling
laws.
This symbol is applicable for EU member countries only.
To avoid potential negative consequences for the environment and possibly human health,
care should be taken while disposing of (i) for EU member countries - in accordance with
WEEE (Directive on Waste Electrical and Electronic Equipment), or (ii) for all other countries,
in accordance with local disposal and recycling laws.
The probe cover need to be disposed after the single use to avoid cross-
contamination.
The probe cover disposal after use should adhere to the local authority
guidelines/norms.
6. Contraindications
The B.O.L.T device is not recommended for people with serious arrhythmia, patients implanted
with an electrical device, patients with preeclampsia, atrial fibrillation, peripheral arterial
disease, and patients undergoing intravascular therapy or arterio-venous shunt or people
who received a mastectomy. Consult your doctor during pregnancy, arrhythmia, and
arteriosclerosis. The analyzed results from the devices are not sufficient to make a correct
diagnosis of the patient`s clinical condition. A detailed clinical history of the patient together
with the results of any other tests suggested by a doctor is also required. This device is
contraindicated for any person who is connected to a wearable or implantable electronic
device or instrument, such as a pacemaker or defibrillator. This device is not intended to be a
diagnostic device. Contact your physician if any abnormal values are indicated. Do not use
the device in an MR environment, in an explosive atmosphere, or on neonatal patients. This
device is not defibrillation proof per IEC 60601-1.
7. Product Features
Elegant design enables simple and easy use of the product.
Multiple Platform Support and same application for multiple users.
Secured Cloud application to share and manage trends with health data.

B.O.L.T User Manual
18
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Monitor and share your health trends with the doctor and loved ones.
Safe and convenient to use.
8. Operating Environment
The B.O.L.T device has been designed for use in the hospitals, clinics or directly by the adult
patient at home to monitor the health conditions. The user or the physician is responsible for
ensuring that the device is stored and used in recommended environmental conditions.
9. Prerequisites
Some basic requirements must be followed as mentioned below to ensure better operation
of the product.
Profile of the user should be minimum of 8 years of education.
B.O.L.T device is designed to be operated with applications like Android or iOS and
SDK available for Windows desktop version also which will be provided on request.
The Android OS version of the Smartphone / Tablet should be 4.0 or higher.
The iOS version of the Smartphone / Tablet should be 9 or higher.
10. Using B.O.L.T Application
10.1 Downloading and Installing B.O.L.T application on your mobile
device
1. Download and Install B.O.L.T application from the Play store (Android) or App Store
(iOS).
2. On successful installation, launch the B.O.L.T application on the mobile device.
3. Select Live URL option
oGlobal Sever –For all global users (Default)
oU.S.A Server –For US users
oOthers –To use own customized cloud (Enter your customized server URL).
4. On confirmation, Click save to enter login screen.
5. Click Create New account and choose the Product Name as BT4.
6. On confirmation, Click save to enter signup screen.
7. Create a new user login with your Email ID and password or use guest login.
8. Click on camera icon to add profile image.
9. Enter the name (First name & Last name).
10. Choose Gender, Male/Female/Others and Click Next.
11. Choose Date of Birth, Height, Weight, Region and Click Next.
12. Enter your E-Mail ID, password and confirm password.
13. Click over the Terms and Conditions and User agreement text to read the info (if
required).

B.O.L.T User Manual
19
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
14. Select the Terms and Conditions and User agreement checkboxes and Click create my
account to complete the user registration.
15. On successful completion of user creation, a verification link will be mailed to the user
email ID.
16. On successful verification, User can be able to login.
17. On successful Login, enter near and dear details under settings panel at the left top
corner of the home view.
Use forget password option to reset your password.
Click on the information icons provided in the home screen
options to view the corresponding information.
Launch Screen Login Screen

B.O.L.T User Manual
20
© Copyright 2019, All Rights Reserved. AmZetta Technologies Private Ltd.
Home Screen Result Screen
Above application screens are for general illustration purpose
only. Actual application screens may vary.
You can also login as a guest user while no login account is
available, which might not store the measured vital parameters in the cloud
storage space on the measured parameters.
This manual suits for next models
4
Table of contents