BEACMED evoStim P User manual

evoStim®P
Therapeutic unit !
for perineal reeducation !
by electrostimulation and
biofeedback (pressure)
User guide
of M.D. REF: evoStim P
(Rev. 7-2020)

2
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 3
CONTENTS
1 Description and intended use 5__________
1.1 - Intended use 5____________________________
2 Indications of perineal electrostimulation7
2.1 - Stress incontinence, 7______________________
2.2 - Urge incontinence 7________________________
2.3 - Mixed incontinence 7_______________________
3 Indications of perineal biofeedback 9___
3.1 - Recruiting Biofeedback 9____________________
3.2 - Training Biofeedback 9______________________
4 CONTRA- INDICATIONS 11_______________
5 WARNINGS AND PRECAUTIONS 12_______
6 CHECKING THE PACKAGE 15____________
7 PREPARING THE UNIT 16________________
7.1 - BATTERIES 16__________________________
7.2 – LEAD WIRES 16_________________________
7.3 - CONNECT THE PROBE 17________________
7.4 - PLACEMENT OF THE PROBE 17___________
7.5 - USING THE UNIT 17______________________
8 OPERATION 18_________________________
8.1 - FREQUENT OPERATIONS 20______________
8.1.1 Switch-ON the unit 20_______________________
8.1.2 Switch-OFF the unit 20______________________
8.1.3 Select a program 20________________________
8.1.4 Select the type of probe in use 20_____________
8.1.5 Set the stimulation level 20___________________
8.1.6 Start the session 21________________________
8.1.7 Adjust the stimulation level during the session 21_
8.1.8 - End of the session 21______________________
8.2 - OPTIONAL OPERATIONS 21_______________
8.2.1 Temporarily stopping the session (PAUSE) 21____
8.2.2 Changing the session time (min’) * 21__________
8.2.3 - Modify the frequency (Hz) 22________________
8.2.4 - Display the pulse width (µs) * 22_____________
8.2.5 - Modify the ACTION! time (s.) * 22____________
8.2.6 - Modify the RELAX time (s.) * 22______________
8.2.7 Manual adjustment of the biofeedback scale * 22_
8.2.8 Automatic adjustment of the biofeedback scale *22
8.2.9 - Restore the factory settings 23_______________

4
8.3 - ACCESSORY OPERATIONS 23_____________
8.3.1 Change the backlight intensity. 23_____________
8.3.2 Enable/Disable the audible feedback (the “buzzer”).
23
8.3.3 Replacing the batteries. 24___________________
9 PROGRAMMES with ELECTROSTIMULATION
25
9.1 - Open-circuit safety cutout 26________________
9.2 - The session time 26_______________________
9.3 - Programme URGE 26_____________________
9.4 - Programme STRESS 26___________________
9.5 - Programme MIXED 27_____________________
9.6 - Programme PAIN 27______________________
9.7 - Programme EVOCATED 27________________
10 The BIOFEEDBACK PROGRAMME 30____
10.1 - Valid contraction 31______________________
10.2 - Pre-set parameters of the biofeedback programme
(BFB) 31____________________________________
10.3 - Reset of the biofeedback sensor 31_________
10.4 - How to perform a biofeedback session 32_____
11 Technical features 33________________
The back-light (“BL”) 34_________________________
12 Trouble- shooting table 36______________
13 Labelling and symbols 41______________
14 Cleaning and maintenance 42___________
Cleaning the unit 42____________________________
Maintenance of the unit 42_______________________
Cleaning electrodes and probes 42________________
15 Information for disposal of the product
43
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 5
1Description and !
intended use
evoStim® Pis a therapeutic unit intended for
perineal electro-stimulation and pressure
biofeedback, suitable for direct use by the patient
but also perfectly suitable for professional use,
offering a great ease of use without sacrificing
flexibility and performance.
1.1 - Intended use
evoStim® Pis a unit for electrostimulation and
pressure biofeedback, 1 output channel
(electrostimulation), 1 input channel (pressure), for
professional use or usable directly by the patient
on the advice of a professional operator
(Physiotherapists , Gynaecologists, Urologists,
Physiatrists, Midwives).
Indications: Prevention or treatment of
incontinence, by means of perineal probes or
surface electrodes.
Perineal electro-stimulation is carried out by
means of a vaginal or anal probe, characterised by
a pair of electrodes. The therapeutic goal is the
improvement of voluntary control of the perineal
musculature (in case of stress incontinence) or the
reflex inhibition of the detrusor muscle (in the case
of urge incontinence).
We should not expect immediate benefits, after the
first session. The main results will be obtained
after repeated sessions (at least 30, administered
daily or on alternate days, depending on the
seriousness of the problem). Any amazing
improvements, after the first sessions, should not
induce the operator or the patient to discontinue
the treatment.
This user guide provides information for the safe
use of this equipment and guidance on perineal
electrostimulation and how effective this treatment
can be.

6
This page has been left intentionally blank
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 7
2Indications of !
perineal !
electrostimulation
2.1 - Stress incontinence,
It is frequently due to sphincter deficiency.
Symptoms include leaking of urine, caused by a
strain (such as coughing, rising from a chair, etc),
in absence of detrusor activity. Stress incontinence
is usually treated with relatively high frequency
electrical pulses, from 35 to 100 pulses per second
(p.p.s.) depending on patients and therapist
preference, this exercises the phasic components
of the muscle fibres which provide strong but short
contractions. The treatment should be performed
for about 20 minutes daily starting with relatively
short work periods and gradually building up
endurance by increasing the contraction time as
the muscles strengthen. Pulse widths may be
selected between 100 to 400 microseconds,
depending on the patient.
2.2 - Urge incontinence
It is caused by detrusor instability. Here the most
appropriate frequency is between 5 and 10 Hz,
with a pulse-width of between 250 and 400
microseconds. The treatment is best performed on
a daily basis for the first week, then 2 to 3 sessions
per week for the next 3 or 4 weeks. The therapy
may be conducted at home.
2.3 - Mixed incontinence
It accounts for about 40% of all cases of
incontinence and is characterised by episodes of
incontinence when straining, along with or
alternating with episodes of incontinence due to
detrusor instability causing urgency. Depending on
the predominance of the first or the second kind of
incontinence (stress or urge), one can decide to
use a relatively high frequency for greater effect on
the muscle tone or lower frequencies to give

8
greatest effect on detrusor inhibition. Urge
incontinence usually responds more quickly than
stress so this is usually treated first. Alternatively
two treatments per day, one for urge and the other
for stress may be carried out.
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 9
3Indications of !
perineal!
biofeedback
Perineal Biofeedback (BF) is an active therapeutic
technique consisting in the fine and real-time
graphical visualisation (and/or audible emission) of
voluntary muscle contractions/relaxation by the
patient.
The therapeutic aim is the improvement of
voluntary control of the perineal muscles. The
patient is visually (visual feedback) and
acoustically (audible feedback) made aware of the
contraction level of its perineal muscles
(pubococcygeus and puborectalis), acquired by
means of a silicone balloon integrated in a special
vaginal probe.
The conscious appraisal of the performed work
determines the effectiveness of the BF treatment.
We can identify 2 pathological situations each
corresponding to a biofeedback techniques:
3.1 - Recruiting Biofeedback
The patient has a PC test (test of pubococcygeus)
degree 1 or lower, with "recruitment" problems of
the perineal muscles. The adopted technique will
be called indeed "recruitment biofeedback."
Despite being very low the acquired signal,
corresponding to the performed contraction, the
level can not even reach the first target of the
smallest range and the patient, through repeated
free contractions, will try to find the "key" for more
effectively recruiting the fibres of perineal muscle,
and attaining the central target.
3.2 - Training Biofeedback
The patient has a PC test (test of pubococcygeus)
of 1 or greater, with problems of "control" of
perineal muscles. The patient is able to reach the
central target, even using a medium range (end-of-
scale), but has a poor fine control of muscles such
as not being able to contract "in time" for a sudden

10
increase in abdominal pressure due to coughing or
an effort. The patient, in this case, is "guided" to
perform multiple contractions of ever-increasing
degree, through visual and acoustic stimuli,
rewarding the achievement of higher goals by
increasing the Full-Scale (FS).
Perineal biofeedback is not an alternative
treatment to perineal electrostimulation, but a
complementary treatment, indicated when the
patient, while managing to weakly contract the
perineal muscles, is unable to sufficiently control
them in cases of sudden increases of abdominal
pressure (coughing or sudden efforts).
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 11
4CONTRA-
INDICATIONS
PLEASE READ CAREFULLY:
In the following circumstances, evoStim® must
NOT be used:
•During pregnancy.
•If you have a heart pacemaker or serious heart
rhythm problems.
•If you are driving or operating machinery.
In the following circumstances, evoStim®
Pcan be used with caution:
1. If you have epilepsy, consult your doctor before
using.
2. On children under 12, apply only under medical
supervision.
Apart from the general contraindications
of electrostimulation, we must consider
the following criteria:
Specific contraindications of the
perineal electrostimulation.
absolute:
pregnancy, kidney disease, lower urinary
tract infections, tumours, post-void
residual urine greater than 100 ml,
vesicoureteral reflux.
subjective:
Reluctance of the patient, hyper-
sensitivity to electrical stimulation.
IF IN DOUBT, CONSULT YOUR PHYSICIAN.

12
5WARNINGS AND !
PRECAUTIONS
1. Carefully read the user guide before starting to
use the unit.
2. This user guide is an integral part of the
medical device; store it in a safe and protected
place, possibly together with the device, to
ensure the availability and readability.
3. Only use batteries AAA 1.5Volt Alkaline
(LR03). The use of any other battery may
damage the unit.
4. Remove the batteries when not used for
prolonged periods (leaking battery acid may
irreparably damage the unit).
5. The unit must not be used to treat painful
symptoms of unknown origin or which have
been insufficiently diagnosed.
6. Do not use the device during sleep.
7. Be careful when using the unit on patient with
reduced sensitivity .
8. Keep the device and its accessories out of
reach of children, the non-self-sufficient people
or pets.
9. DO NOT apply electrodes on the throat or
larynx nor over the carotid sinus or the sides of
the neck, (the area of heartbeat detection).
May increase the risk of abnormalities of blood
pressure or heart rhythm.
10. DO NOT place any surface electrodes for
stimulation in TRANS-THORACIC WAY. The
application of the electrodes close to the
thorax may increase the risk of arrhythmias or
cardiac fibrillation.
11. DO NOT place any surface electrodes for
stimulation in TRANS-CEREBRAL WAY. It
could cause symptoms such as dizziness,
nausea, vomiting, headache.
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 13
12. Do not apply the electrodes on the eyelids or
around the eyes. It could affect the intra-ocular
pressure
13. DO NOT place electrodes on/in the mouth. In
case of inappropriate contractions may
increase the risk of suffocation.
14. Avoid placing surface electrodes over any area
affected by acute phlebitis.
15. DO NOT use the unit at a distance lower than
3 metres from any high frequency therapy unit
(short wave or microwave) or close to a
microwave oven.
16. DO NOT use the unit at a distance less than
those indicated in the table on page 40,
respect to a radio frequency communication
device (RF transmitters, mobile phones,
remote controls).
17. DO NOT the unit on a patient in which it is
used simultaneously an electrosurgical high
frequency device. It may increase the risk of
instability of the device and / or burns under
the electrodes.
18. Do not use the device on a patient on which a
monitoring instrument for physiological
parameters (such as ECG or others) is used
simultaneously. It could be affected by
electrostimulation.
19. The equipment can deliver electrical pulses
with a current density higher than 2mA r.m.s./
cm2.
20. Store the unit and accessories in the pouch for
storage and transportation.
21. Avoid violent impact and any improper
solicitation of the unit.
22. Do not expose the unit or accessories to
temperature levels higher or lower than those
recommended in the technical characteristics.
23. DO NOT use the unit in an ambient
temperature above or below the recommended
operating range.
24. DO NOT touch the unit in any way with wet
hands, in order to prevent possible penetration
of liquids.

14
25. Keep the unit dry and protect from
condensation.
26. If in doubt whether liquids have penetrated
inside the unit, it is advisable not to use the
instrument and to send it to the manufacturer
for testing.
27. Prevent the formation of condensation due to
thermal sudden change.
28. In presence of condensation, avoid switching
on the unit because it could be damaged.
29. In case of an evident or suspected defective
operation of the unit, the user is advised to
send the unit to a BEACMED authorised
technical after sale Servicing Centre, for
testing.
30. No repair or modification of this device or its
accessories is allowed unless previously
authorised in writing by the Manufacturer.
31. Avoid using the unit on more than one patient
per session.
32. Use only original accessories, if supplied. If the
device is used with commercially available
probes, they must be CE marked, as a class
IIa medical device, according to Directive
93/42/EEC MDD. Before using these special
accessories, it is mandatory to carefully read
the instructions for use and cleaning, which
must be included in their packaging.
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 15
6CHECKING THE!
PACKAGE
The therapy unit evoStim® Phas been designed
for a friendly but effective use. Before using it, you
should carefully read the chapters: 4 -
CONTRAINDICATIONS and 5 - WARNINGS and
PRECAUTIONS.
PERSONAL USE OF THE PROBES!
Do not use the perineal probes (vaginal or anal)
on different patients. The probes are for personal
use. This is to prevent the transmission of
venereal diseases or other more serious
diseases.
The evoStim® Ppackage should contain the
following parts:
After verifying that the contents correspond to
what is listed here, you can proceed to prepare
your unit for the session.
Q.ty
Code
Description
1
EVO-P
Unit evoStim®P
1
BAT/LR03-03
Kit 3 AAA alkaline batteries 1.5 V. (LR03)
1
ESTIM-KEY
Plastic key for battery compartment
opening.
1
CV/evoStim_kit_P
Gray bipolar cable with protected 2mm
banana termination and mini axial
connector. Length 99cm.
1
ESTIM-SUPP-PGB
interlocking stand for vertical support of
the unit on a horizontal surface
1
evoPouch
PVC carrying bag with necklace (IP02)
1
EStim_bag
Padded bag or rigid plastic handbag
1
ISTRU-evoStim P
User manual for MD evoStim P.
1
Sonda perineale
The probe Periprobe VAG-2STFW is
provided, unless otherwise requested by
the customer.

16
7PREPARING THE!
UNIT
7.1 - BATTERIES
To remove the battery-compartment cover, insert
the special plastic key provided
in the slot on the side of the
cover and pushing in the
direction of arrow ⓐ (DO NOT
turn the key!); Lift off the battery
cover; Insert the three batteries
supplied ⓑ, observing the
polarity shown on the bottom of
the battery compartment ⓒ.
Close the battery-compartment
with the
cover ⓓ.
Note: The unit may not work if one or more
batteries are inserted in reverse.
CAUTION!: There is a risk of explosion if the
batteries are fitted incorrectly. Replace only with
AAA Alkaline 1.5 volt batteries (LR03). Do not use
other batteries. Do not mix old and new batteries.
Do not dispose of the batteries in a fire and keep
them out of reach of children. The batteries must
be removed from the device before it is scrapped
and disposed of safely. When the unit is not used
for a long time, you must remove the batteries
to avoid deterioration and leaking battery acid. This
could irreparably damage the unit’s electronics.
7.2 – LEAD WIRES
Unravel the gray lead wire
and insert the plug(s) into
either of the outlets, located at
the base of the unit. If only
using one lead, insert into the
CH1 outlet as marked on the
unit (.
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)
ⓑ
ⓒ
ⓓ
ⓐ

evoStim® P- User guide 17
7.3 - CONNECT THE PROBE
Remove the probe from the
bag, rinse under tap water if
it is a new probe, then
connect to the leads. Each
lead wires should be
connected as shown in the
picture or according to the instruction leaflet
included in the package of the probe. Also read
paragraph 7.6.
If you use the balloon probe, connect (always
before inserting the probe) also the tube that
comes out of the probe to the corresponding tube
that comes out of the equipment (Bf-P) ㉕. (only in
the EVO or BIOFEEDBACK program).
7.4 - PLACEMENT OF THE PROBE
Moisten the insertable part of the probe with tap
water or water based gel, to improve the
conductivity of the electrodes. Gently insert the
probe in the vagina (or anus), according to the
instructions included in the package of the probe.
7.5 - USING THE UNIT
Read the chapter 8 (OPERATION) and use the
unit according to the therapeutic aims.
7.6 - TYPE OF PROBE VS- WAVE-SHAPE
The stimulation output must be connected, by
means of the included lead wire, to a probe
(vaginal or anal). The cables have two endings to
2mm plug, a red and a black colour. Using
“symmetrical bi-phasic pulses” ( ), the greater
effect will be felt at the electrode connected with
the RED plug.
If the waveform is selected with “bi-phasic
alternated pulses” ( ), there will be no
predominance of any of the two electrodes.
In general terms, consider the followings:
• if the probe has ring-like electrodes, the RED
output of CH1 lead must be connected to the RED
connector of the probe and the suitable wave
shape is “symmetrical bi-phasic pulses” ( ).
• if the probe has lateral electrodes, the suitable
wave-shape is “bi-phasic alternated pulses” ( ).

18
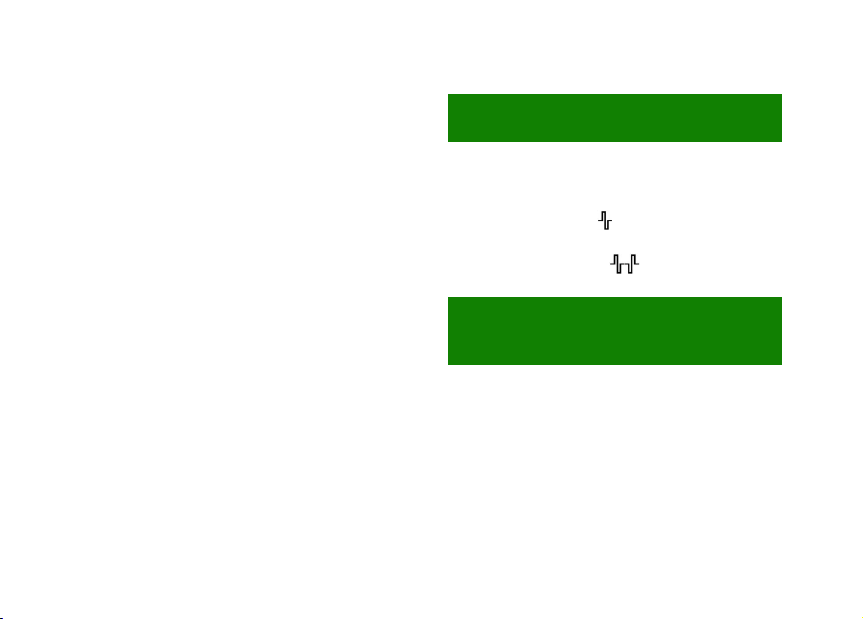
8OPERATION
The ergonomics of evoStim® Punit is based on
the rotation of the upper knob and the touch
screen. Through the knob it is possible to set/
modify the stimulation intensity or the value of the
various parameters; through the push-button,
integrated in the knob, you can switch-ON/OFF or
pause the unit. Using the touch screen you can
select the programmes and variables to change.
①- Upper knob with integrated push-button
②- Parameters of the program (3 digits)
(§ 8.2.2, § 2.2.3, § 8.2.4, § 8.2.5, § 8.2.6).
③- Touch-area ACTION! (§ 8.2.5).
④- Touch-area RELAX (§ 8.2.6).
⑤- Touch-area buzzer enable/disable (§ 8.3.2).
⑥- Centre of the Concentric-Circles target (CCT)
(§ 8.2.8).
⑦- Stimulation intensity (2 digits) (§ 8.1.5-8.1.7).
⑧- Wave-shape (type of impulse) (§ 8.1.4).
⑨- Low battery indicator (§ 8.3.3).
⑩- Pause state indicator (§ 8.2.1).
⑪- Touch-area reset biofeedback input (§ 10.3).
⑫- Target zone of the linear bar-graph (§ 10.1).
⑬- Linear Bar-Graph (BG) (§ 10.1).
⑭- Multi-Circles target (CCT) (§ 10.1).
⑮- Lock state indicator (§ 8.1.7).
⑯- Touch-area backlight level (§ 8.3.1).
⑰- Touch-area programme URGE (§ 9.3).
⑱- Touch-area programme STRESS (§ 9.4).
⑲- Touch-area programme MIX (§ 9.5).
⑳- Touch-area programme PAIN (§ 9.6).
- Touch-area programme EVO (§ 9.7).
- Touch-area programme BIOFEEDBACK
(§ 9.7, § 10.1, § 10.4).
- Full-scale biofeedback (3 digits)
(§ 8.2.7-8.2.8).
- Stimulation output connector (§ 7.2).
㉕- Pressure input (from the balloon) (Bf.P).
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)

evoStim® P- User guide 19
Functions of the upper knob: Press and hold the knob
downwards to SWITCH ON or SWITCH OFF, simply
press to START THE SESSION or PAUSE it. Turn the
knob to INCREASE or DECREASE the selected
parameter or to UNLOCK the controls during the session.
⬇ON/OFF
DECREASE
⤵INCREASE
②
③
⑤
④
⑥
⑦
⑧
⑨
㉓
⑪
⑬
⑭
⑮
⑯
⑰
⑱
⑲
⑳
㉑
㉒
⑩
㉔
㉕
①
⑫
}
20
8.1 - FREQUENT OPERATIONS
Before carrying out the following operations,
connect the probe to the device at least through
the gray cable Ch.1 (24) and insert the probe into
the vagina or anus (in the case of anal probe). For
the EVO and BIOFEEDBACK programmes, the
probe balloon is also used, which requires the
connection of the pneumatic tube to the Bf. P input
㉕(see also § 10.4.1).
8.1.1 Switch-ON the unit
Press for 2 seconds the button integrated in the
knob ①.
8.1.2 Switch-OFF the unit
Press for 2 seconds the button integrated in the
knob ①.6The6unit6will6also6turn-OFF6if the session
does not start in 5 minutes.
8.1.3 Select a program
Touch one of the 6 rectangular areas at the lower
side of the display (, , , , ㉑o ㉒).
The label of the selected program will appear in
reverse.
Every time the unit is turned ON, it will
automatically load the last used programme.
8.1.4 Select the type of probe in use
By touching the area ⑧, you can select the wave-
shape according to the type of the probe in use:
•the simple impulse ( ) for the probes with ring-
like electrodes;
•the alternated pulse ( ) for the probes with
lateral electrodes.
The choice of the type of probe used in the last
session, will be automatically loaded when the
unit is switched-ON.
8.1.5 Set the stimulation level
If a program providing electrical stimulation has
been selected (URGE-STRE-MIX-PAIN-EVO),
after connecting and positioning the probe, tap the
area 0mA ⑦, then turn the knob clockwise to feel
a strong and comfortable level of stimulation.
After adjusting the stimulation level, it is required
to start the session (§ 8.1.6), otherwise the
BEACMED s.r.l. - Via Monte Bianco, 12 - 27040 Portalbera PV (Italia)
Table of contents
Other BEACMED Measuring Instrument manuals
Popular Measuring Instrument manuals by other brands

Hanna Instruments
Hanna Instruments HI9813-51 instruction manual

Deif
Deif BRW-2 quick guide

GHM-Messtechnik
GHM-Messtechnik GREISINGER GTF 111-Ex Series operating manual

Shimpo
Shimpo DT209X instruction manual

Hanna Instruments
Hanna Instruments HI 84429 instruction manual

Amprobe
Amprobe TIC 300 PRO user manual













