BELIMED WD425E User manual

User
Operating Manual
WD425E
Copyright F. Gehrig AG Ballwil,
Switzerland 2005
Art. Nr. 78563_E

Operating Manual WD 425E
Rev. 1.1, LS Page 2 of 34 78563_E rev 1.1.doc
TABLE OF CONTENT
!IMPORTANT NOTICE! ................................................................................4
!CAUTION! .............................................................................................................................4
!WARNING! ............................................................................................................................5
1. General Directives ref. Medical Products Processing............................................7
1.1 Pre-treatment of Medical Product ............................................................................................................. 7
1.2 Notes ref. orderly Cleaning ....................................................................................................................... 7
1.2.1 Influence of time and temperature .................................................................................................. 7
1.2.2 Impact of Water Quality .................................................................................................................. 8
1.2.3 Appropriate Rinse oriented Loading ............................................................................................... 8
1.3 Flexible Endoscope Cleaning ................................................................................................................... 8
1.3.1 Pre-cleaning and Function Check................................................................................................... 9
1.3.2 Placing of water tight, flexible Endoscopes................................................................................... 11
1.3.3 Leak Test for Endoscopes............................................................................................................ 12
1.3.4 „Manual“ Leak Test....................................................................................................................... 12
1.3.5 Automatic Leak Test..................................................................................................................... 13
1.3.6 After Endoscope Processing ........................................................................................................ 13
1.4 Instrument Rack...................................................................................................................................... 14
1.5 AN Rack ................................................................................................................................................. 14
2. Operation, Keyboard ...............................................................................................15
3. Operation Indication................................................................................................15
3.1 Normal Operation ................................................................................................................................... 15
3.2 Program Start ......................................................................................................................................... 16
3.3 User Identification ................................................................................................................................... 16
3.4 Rack Detection ....................................................................................................................................... 17
3.5 Identification Batch Content.................................................................................................................... 17
3.6 Endoscope Identification......................................................................................................................... 19
4. Program Launch and Process Sequence..............................................................20
•Suppressing wash liquid drainage according to ∫18 ISSG Infection Protection Law (BGA / RKI) 21
5. End of Service Procedures .....................................................................................22
6. Error Message without Process Interruption........................................................22
6.1 Dosing Additive Container empty............................................................................................................ 22
6.2 Door Locking........................................................................................................................................... 23
6.3 Sensor Difference................................................................................................................................... 23

Operating Manual WD 425E
Rev. 1.1, LS Page 3 of 34 78563_E rev 1.1.doc
6.4 Maintenance Prompt, Maintenance Frequency ...................................................................................... 23
6.5 Load Cut-off (external)............................................................................................................................ 24
6.6 The Machine does not wash properly ..................................................................................................... 24
7. Machine does not run..............................................................................................24
8. Error Indication at Time of Process Interrupt .......................................................25
9. Activate Modem Connection...................................................................................32
10. Wash Chamber Cleaning and Maintenance Assignments...................................32
10.1 Daily Maintenance .................................................................................................................................. 32
10.2 Annual Qualification Renewal ................................................................................................................. 32
11. Manufacturer, Maintenance and Service ...............................................................34
Edition

Operating Manual WD 425E
Rev. 1.1, LS Page 4 of 34 78563_E rev 1.1.doc
!IMPORTANT NOTICE!
Be sure to carefully read and understand this manual prior to commissioning the automat.
Limitations of Application
The manufacturer does not assume any responsibility for damages resulting from improper application of
the machine, such as operating errors, unintended application or operation by untrained personnel as well
as failure to follow instructions found in this manual. Modifications of the automat, especially technical
modifications by unauthorized personnel – without written consent by the manufacturer - will result in total
loss of all warranty claims. Product liability will be annulled.
The cleaning automat is designed exclusively for the range of applications listed in the user manual, i.e.
reusable medical products.
Operation of this machine is designed only for indoor operation such as laboratories and hospitals. Appli-
cation for textile materials may result in damage to the machine. The operation must be handled by prop-
erly instructed and trained personnel.
!CAUTION!
Cleaning and disinfection performance
A cleaning assignment is assured only if
• a validation of the program parameters such as dosing and appropriate assignment of media has
been completed by qualified personnel,
• the wash arms are permitted to freely rotate,
• wash good racks have been properly loaded,
• the position of loading rack docking devices match those of the machine,
• original load racks of the respective machine are used (no foreign products),
• coarse sieves and wash arms are all in place and are regularly cleaned,
• scheduled maintenance operations are correctly carried out by technical personnel.
Special note: clean filters and dosing pumps must be regularly serviced and checked.
Validation
The top goal of process validation in the realm of the statute for medicinal products is to assure a high de-
gree of safety in processing medicinal products, thus protecting the health of the patient and the machine
operator. The initial validation always consists of these elements: acceptance procedures, functions and
performance assessment.

Operating Manual WD 425E
Rev. 1.1, LS Page 5 of 34 78563_E rev 1.1.doc
!WARNING!
The user is responsible for checking the cleanliness of instruments following each cleaning run of the ma-
chine.
Safe sterilization of medicinal products is possible only if they are clean. It is, therefore, mandatory to scru-
tinize the effect of cleaning. Following cleaning / disinfecting, no soiling remnants (e.g. encrustations,
films) must be discernible by normal eyesight.
The full disinfection program must not be interrupted at any time or for any reason. After each program
stop, the program must be reset and restarted by means of the „I / O“ (ON - OFF ) button.
If the disinfecting process must be interrupted due to a dangerous situation, the wash chamber door may
not be opened for about 5 minutes. Escaping vapors may be a health hazard.
At the end of the drying process, metallic wash goods may be very hot and may lead to serious burns,
when extracting the wash goods without the use of gloves.
During replenishing containers of cleaning and disinfecting additives, eyes, hands and clothing must be
protected. Gloves must be worn when handling additives, such products must not be ingested, prevent eye
and skin contact!
Check if the appropriate additive is being replenished or exchanged.
Dosing must be verified following each container exchange.
Detergents ad their viscosity is influenced by ambient temperature, possibly leading to over or under dos-
ing.
The machine must be immediately shut down if
• Smoke generation is observed,
• Water is escaping from the machine,
Contact Tech Service without delay.
Cut electric power to the machine before carrying out any maintenance operations.
Sheathing
The machine may be operated exclusively with all metal sheathing in place. An uncontrolled discharge as a
result of pipe conduit burst might lead to serious scalding by hot water. Be aware that water may have
temperatures up to 93°C.
!Warning!:
Only authorized personnel may open the service door to the electro cabinet or remove any
sheathing. Upon removal of sheathing or opening the electro cabinet, it is possible to
come in contact with unprotected contacts of tank heater, boiler heater, dryer heater, re-
lays, transformers, valves and control unit. This represents a health hazard, even lead to
death.
Prior to opening the electro cabinet or removing machine sheathing, it is mandatory to
turn electric power off by main switch or connector plug. Above listed risks can be
avoided only by heeding the above warnings.
Operating Manual WD 425E
Rev. 1.1, LS Page 6 of 34 78563_E rev 1.1.doc
!Caution!:
If additional components are connected (dosing pumps, external printers etc), be sure that
cables cannot be accidentally pulled (provide pull relief).
Forceful pulling on components may lead to electric shock! Prior to installation of addi-
tional equipment or replacement of components, machine must be rendered powerless!
!Caution!:
Only original parts may be used for repairs and optional component installations. Installa-
tion of any foreign parts will result complete loss of warranty claims and annul the product
liability provisions.

Operating Manual WD 425E
Rev. 1.1, LS Page 7 of 34 78563_E rev 1.1.doc
1. General Directives ref. Medical Products Processing
1.1 Pre-treatment of Medical Product
Basically, all outer and inner surfaces to be treated must be accessible for the decontamination, disinfection
and sterilization liquids (open valves / faucets, linkage instruments!). Special attention is required for the lu-
mens. Complex medical products may require disassembly. Prior to processing, MIC instruments must be
disassembled according to manufacturers’ instructions. Baked on remnants are extremely critical for instru-
ments used in operative endoscopies, as removal of grime remnants found in narrow lumens are hard to
remove by machine mechanical processes. Advice by manufacturers of medical devices must be heeded
(DIN EN ISO 17664, requirements information to be made available by the manufacturer referring to the for-
mation of medical products, designed for sterilization).
Course soiling of any medical product must be removed immediately upon application. Remaining of dried
blood and tissue must be prevented by establishing suitable procedures (e.g. wiping of external soiling and
rinsing of channels immediately upon application). This is required especially for the optimal prevention of
performance curtailment (drying of viruses in protective colloids).
Ways and means of pre-cleaning must be adapted for the subsequent reconditioning processes and must
not impede them (fixation of blood and proteins, foam formation in the CDM).
Impediment of decontamination is given by:
• Pretreatment with aldehyde disinfection media
• Pretreatment with alcoholic solutions
• Dropping antiseptic solutions on wash goods
• Aldehyde and alcohol vapors
• Heat pretreatment
!All such treatments act in the sense of fixing proteins and may e.g. contribute to the conservation of prion in-
fectiousness !
See recommendations by the RKI „Requirements for hygiene in processing medical products. Federal Health
Flyer 44 (2001) : 1115-1126”
1.2 Notes ref. orderly Cleaning
1.2.1 Influence of time and temperature
In the selection of process and program parameters such as temperature, time and additives, keep the fol-
lowing in mind:
Chemistry and mechanics need time to develop their effectiveness. One should never be skimpy with regard
to time used in cleaning.
Initial high temperatures at the beginning of a cleaning process may fix proteins. It is for that reasons that a
cleaning process should always start with a generous, cold pre-rinsing cycle. Cold pre-rinsing should not be
too short, in order to prevent any denaturing of proteins. After all, freedom from proteins also means ab-
sence of prions.
Alkaline cleaning is characterized by high efficiency pertaining to dissolution of protein and fat remnants. On
the other hand, it may have the disadvantage of altering materials. Under this aspect, it may pay off to select
medical products that can tolerate alkaline cleaning.
For alkaline cleaning, this rule of thumb applies: augmentation of cleaning temperature by 10 °C results in
doubling the cleaning performance. In fact, alkaline detergents are capable of developing their protein-

Operating Manual WD 425E
Rev. 1.1, LS Page 8 of 34 78563_E rev 1.1.doc
splitting, hydrolytic effect only at elevated temperature levels. In case high temperatures are not feasible for
reasons material properties, then an improvement of performance may be obtained by extending the clean-
ing time
See Belimed Factory Programs.
1.2.2 Impact of Water Quality
Water quality has a significant influence on cleaning efficiency. Raw water (CW and WW) must at least meet
the criteria of drinking water. Inferior water quality may bring about problems such as:
• Calcification
• Corrosion
• Filming and discoloration
• Water stains
• Reduced cleaning performance
The water quality for the various steps in processing, such as CW, WW, DI water, must be defined and re-
quires monitoring. The quality of the available water must be taken into consideration when dosing additives
are selected.
Aside from the chemical qualities of water, its microbiological properties must be kept in sight as well.
Considering the special nosokomial risk, the regular chemical analyses of the water must be expanded by
micro-biological investigations to keep opportunistic and ill-making germs in check.
1.2.3 Appropriate Rinse oriented Loading
Influence of loading properties on cleaning performance
Proper loading of wash goods has a significant influence on cleaning results. Therefore, the following sug-
gestions deserve your attention:
• Instruments with hollow spaces, shanks, hoses, respiratory tract systems must be thoroughly flushe on
the inside too. This requires special gadgets that are adapted for the various instruments, e.g. AN rack
featuring a flushing rig.
• Overturning of repositories such as container lids or light weight bowls must be prevented as they might
cause significant water remnants and thus promote lye contamination. Also, drying performance would
be impaired.
1.3 Flexible Endoscope Cleaning
Note:
DI water supply lines do not feature a free flow section on the machine side to prevent in-
festation.
!Caution!:
For the purpose of cleaning flexible endoscopes, approved detergents and disinfecting
additives must be used.
When performing pre-cleaning chores of endoscopes, appropriate protective clothing
must be worn, thus to prevent infection by contaminated endoscopes.

Operating Manual WD 425E
Rev. 1.1, LS Page 9 of 34 78563_E rev 1.1.doc
1.3.1 Pre-cleaning and Function Check
Immediately following endoscopy, all channels must be flushed in order to assure permeability. Surface-drying in
excess of 15 minutes must be avoided.
Instructions and operating provisions by the manufacturers must be followed.
In the recommendations by the RKI, see „Requirements for Hygiene in Processing flexible Endoscopes
and endoscopy auxiliary Instruments “ the following is suggested (excerpt).
Federal Health Flyer 2002- 45:395-411 (Springer Publishers 2002)
Machine borne processing with decontamination and disinfection units
1 Pre-cleaning
• Carry out pre-cleaning immediately following application.
• Already at the time of endoscope removal following the investigation, use a soft towel to wipe the insertion part,
thus removing coarse contamination objects.
• Dip distal end in a receptacle containing cleaning fluid, alternating operation of suction and air-water valves (if
feasible, operate cleaning valve). Suck cleaning fluid and air through the endoscope channels, thereby check-
ing for permeability and function of all channels.
• Finally, suck channels clean by air.
• Separate endoscopes from optical rinsing system, supply suction hoses as well from light source to bring them
to the processing area (haul in closed containers / bowls with lids).
2 Leak Test
• Attach water protective cap on video scopes to guard electrical contacts.
• Deposit endoscope in a bowl containing cleaning solution.
• Remove all valves and distal caps, place them in cleaning solution.
• Carry out leak test as per manufacturers’ instructions.
• In case of positive results of leak test (confirmed perforation), endoscope may not be further processed. The
outer mantle must be wiped with instrument disinfectant, i.e. Isopropanol 70% (is permitted by the manufac-
turer), the channels dried with compressed air, the endoscope wrapped in a protective film wrap, packed in
shipping case and handed over to the service station under marking „untight, not disinfected “.
3 Manual cleaning
• Mix up cleaning solution according to manufacturers’ instructions.
• Following leak test, immerse completely in cleaning solution.
• All cleaning steps must be carried out below the cleaning solution’s surface; this to prevent splashing of con-
taminated liquid.
• Use a lint-free one way towel to wipe the outer mantle of the endoscope.
• Use a suitable, soft brush to clean channel and valve openings, distal end and control elements.
• Bring Albaran lever of Duodenoscopes to middle position and use a suitable, soft brush to clean on al sides.
• For mechanical brush cleaning, use suitable, disinfected, flexible brush to brush clean all accessible channels
systems repeatedly until brush is pulled through without any new contaminants. Clean all valves and distal caps
using a soft brush.
• Using equipment specific adapters, connect all channels and rinsing connectors then flush with cleaning solu-

Operating Manual WD 425E
Rev. 1.1, LS Page 10 of 34 78563_E rev 1.1.doc
tion, thus removing all dissolved particles.
• Clean cleaning brushes and disinfect along with endoscope.
4 Flushing off cleaning solution
• Place endoscope and accessories (valves and cleaning brushes) in a bowl filled with clean line water, then flush
all channels such as to remove cleaning agents.
• Blow out all channels with compressed air.
5 Preparation of cleaning and disinfecting machine (CDG-E)
• Following manufacturer’s instructions, place cleaned endoscope in receptacle rack of the CDG-E, if feasible
connect endoscope with respective system.
• Place accessories (e.g. valves, distal caps, cleaning brushes) in accessories basket.
• Push receptacle rack into CDG-E unit, close door, select program, launch CDG-E.
6 Removal of endoscope from CDG-E unit
• Use disinfected hands or fresh one-way gloves to remove endoscope.
• Carry out function test with endoscope.
• If applicable, post-dry electric contacts and channel systems with compressed air.
• Subsequently, endoscope is ready for renewed application with patients.
• Storage of endoscope must be done in totally dry, dust protected, preferably in suspended position in an endo-
scope cabinet. Store valves dust-free and dry.
• Do not insert valves in endoscope for storage.
This completes the excerpts of recommendations according to the Federal Health Flyer 2002- 45:395-411
(Springer Publishers 2002).
Additional items of importance:
Pre-cleaning
Pre-cleaning and leak test must be carried out in disinfected cleaning solution immediately following endoscope
application. Clean outside surface of endoscope with gauze, i.e. wipe.
Endoscope channels must be pre-cleaned according to manufacturer’s instructions using approved cleaning
materials (e.g. brushing).
Function test
According to function, flush and suck channels with disinfected cleaning solution, then blow out with air (a low
foaming, machine compatible cleaning agent must be used). Permeability check of the individual channels is
mandatory.
Important Note !
The manual leak test performed by operating personnel, prescribed by the manufacturers must be carried out in
spite of the automatic leak test performed by the machine according to program in mechanized processing.

Operating Manual WD 425E
Rev. 1.1, LS Page 11 of 34 78563_E rev 1.1.doc
1.3.2 Placing of water tight, flexible Endoscopes
First, remove valves, distal caps etc.
Open pressure chamber cassette.
Insert distal end of endoscope in spiral shaped Teflon hose until the hand part can be placed in wash box.
Position hand portion of endoscope such as to have supply end fit in the respective notch of the wash box, then
lock in position.
Carefully close pressure chamber cassette. When closing lid, be sure to prevent any damage to the supply end.
Place supply end on wire spiral helix.
Connect adapter with supply components for leak test. For this, on endoscope side, the connection used for the
manual leak test is to be used. The other end of the adapter hose is connected on machine side in the wash
chamber at the ceiling, right and left side respectively.
The supply end is in-
serted in the cutout of
the endo box, held in
place and cover care-
fully closed.
The pressure test
adapter is connected with
the appropriate location
in the wash chamber.

Operating Manual WD 425E
Rev. 1.1, LS Page 12 of 34 78563_E rev 1.1.doc
In case of single endo-
scope processing, the
shown closure plug is
installed in the empty
endo box.
After inserting the plug,
close box cover.
Caution
In case a disinfection program is interrupted, the endoscopes are not properly disinfected. The program
must, therefore, be repeated.
Important
The seal system on endo box and coupling must be checked periodically. Also a mandatory check pertains
to the pressure chamber and its closure.
1.3.3 Leak Test for Endoscopes
During the wash phase, the test pressure is continuously monitored. Even the most minute deviations are
judged as errors and are shown on the display.
Leak test procedure:
Phase 1 - 4 are carried out prior to the first wash cycle and is performed without “water contact”.
Build-up of test pressure.
Setting phase: 10 seconds. Air disperses evenly within the endoscope.
Test phase. Holding inside pressure in the endoscope within a predetermined range at a predetermined
test pressure threshold. If target values are not attained, control system cuts off program. Display shows
error code 170 - 179.
Rinsing phase with endoscope protection: during the wash and rinsing phase, endoscope inside pressure
is controlled to always be above the external water pressure.
Venting phase: The endoscope is being vented.
1.3.4 „Manual“ Leak Test
If door is open (S110), the leak test may be initiated by pressing door key for 1 second. Instead of „Program
ready, door open“, display shows „Endoscope Leak Test“. The door key blinks for the duration of the test. In-
terruption of the test is possible only by means of the door key or with the IO button. If the leak test is deacti-
vated in the setup, this function is completely eliminated.
Endoscope Leak Test

Operating Manual WD 425E
Rev. 1.1, LS Page 13 of 34 78563_E rev 1.1.doc
L●●●●●●●IR ●●●●●●●I
The gradually increasing test pressure is depicted optically by dots. Each dot corresponds to a given pres-
sure level, see chart below.
L ●●●●●●I R ●●●●●●●I
280 start 100 150 200 230 260 280 full start 100 150 200 230 260 280 full
200 start 50 100 130 150 180 200 full start 50 100 130 150 180 200 full
Filling phase
Selected holding
pressure
normal
280 mbar
normal
280 mbar
reduced
200 mbar
reduced
200 mbar
Test pressure 280 mbar 2.74 V 230 mbar 2.34 V
The endoscope is pressurized to target pressure of 280 / 230 mbar (relative). If the target pressure of 280 /
230 mbar (relative) not attained within 10 minutes, display shows error 170 or 175.
Setting phase
Upon reaching test pressure of 280 / 230 m bar, 10 seconds of dwell time is given to allow stabilizing. At end
of dwell period, all dots disappear momentarily and the „I” at the end of the dot row are shown in blinking
mode. At this moment, the automatic leak test is launched.
Test:
For the duration of the test, the “I” keeps blinking. Depending on setup calibration, a waiting period of 30 or
60 seconds is observed. Should pressure drop by more than 2.5 m bar per minute, i.e. 52 mV, error 171 or
176 is shown. As the pressure drops, the dots disappear according to pressure values.
Venting (applies also after endoscope protection)
The endoscope is being vented. In case the endoscope cannot be brought to a value below 1.5 V within 10
minutes, error 174 / 179 appears.
If test is interrupted either by the IO or the door key, venting is initiated also. The display returns to “Program
ready”. Blinking of door key ceases.
1.3.5 Automatic Leak Test
The automatic leak test is always and only performed at the beginning, after program start. Once the pres-
sure test is completed, the actual wash process may be launched. The descaler rinsing and the filling proc-
ess, however, may be started already but must remain until after the pressure test has been completed.
Filling and pressure test and the filling process is indicated on the display analog to the manual leak test, and
the door button blinks also. The indication „Descaler rinsing“ is shown only at the beginning.
If the test is deactivated in the setup, then this function is completely dropped.
1.3.6 After Endoscope Processing
Once the endoscope is removed from the machine and the rack, small quantities of remnant water may be
found in the endoscope. To prevent germ formation in the endoscope channels, they must be completely
blown out.
It is recommended to store endoscopes in a drying cabinet.

Operating Manual WD 425E
Rev. 1.1, LS Page 14 of 34 78563_E rev 1.1.doc
1.4 Instrument Rack
• Prerequisite for an effective machine
cleaning is appropriate and rinsing
oriented loading of the racks.
• Articulated instruments must be open.
• The sieve trays must not be over-
loaded.
• The spray arms must be able to al-
ways freely rotate
• Large surface instruments must
placed on sieve trays that they do not
encumber the cleaning of other in-
struments with their spray shadow.
• Components of motor system may
only be machine processed if the
manufacturer has approved such han-
dling in connection with special proce-
dures and retainer equipment. Simple
tools and accessories may be proc-
essed just like surgical instruments.
1.5 AN Rack
Inappropriate loading (improperly placed) anes-
thesia hoses may tear loose from their retainer
and thus impede the wash arm in its rotation.
The disconnected respiration hose will thus be
inadequately rinsed and will remain unclean.
The drying process is prolonged, is delayed if
water remnants found in water sacks of hoses
cannot be drained
Effect of rack loading characteristics on dryer performance
Critical areas such as flanges of containers, OP shoe heels, water sacks in hoses, turned over bowls, im-
permeable hollow places of instruments, where water remnants cannot be completely drained and hence
must be evaporated.
Operating Manual WD 425E
Rev. 1.1, LS Page 15 of 34 78563_E rev 1.1.doc
If at all possible, wash good must be self draining. The supplementary heat energy and the prolonged drying
cycle required to dry these spots is disproportionately high and it promotes aging of equipment components.
When offloading wash goods, the trapped water is drained; metallic wash goods will dry within a few min-
utes.
When in doubt, do not hesitate and contact your Belimed medical consultant.
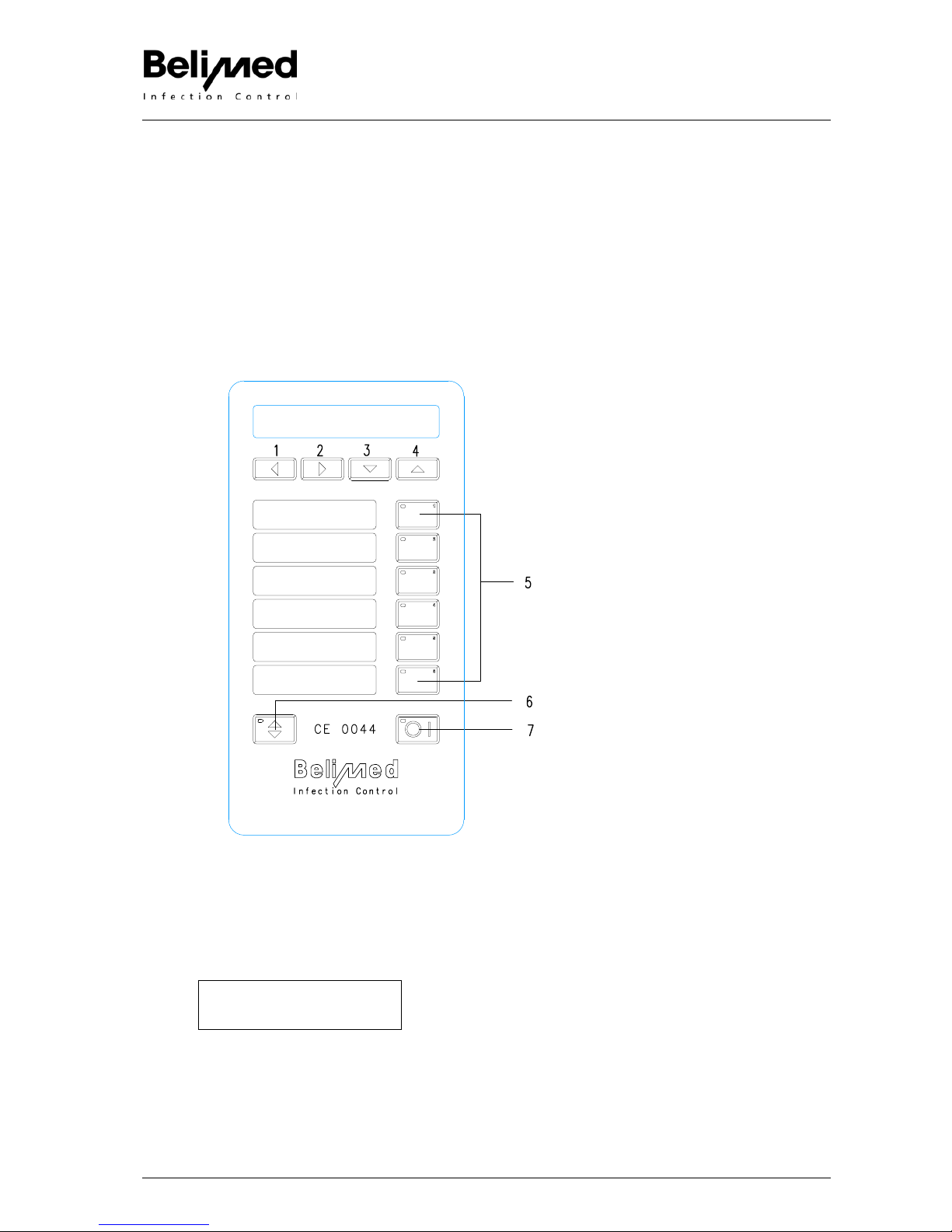
2. Operation, Keyboard
Pic. 73091
Legend
1- 4 Arrow keys
1. Printout Operating Data is like program
recipe and setup calibration.
2. Beeper ON / OFF, at program end and in
case of error, an acoustic signal is
sounded.
3. Printer ON / OFF
4. Shift key programs P7 - 12
Program keys (PT) 1, 2, 3, 4, 5, 6
5. Program keys, selecting of programs P 1-
6, with shift P 7-12
6. Door button, open door
7. Reset, i.e. ON / OFF button (IO)
Pressing for 4 seconds, then releasing,
batch number and machine N° is dis-
played.
3. Operation Indication
3.1 Normal Operation
Close the door. Display shows the following:
Program ready
Date Time
Check if date and time are correctly indicated, required corrections can be made by a trained house techni-
cian. The data are passed on for batch documentation. The machine is operational and may be started by
means of program keys P1 to P6.

Operating Manual WD 425E
Rev. 1.1, LS Page 16 of 34 78563_E rev 1.1.doc
3.2 Program Start
The machine may be launched by program keys P1 to P12 or by automatic program detection on the rack
(Configuration ON). With automatic program recognition, any program may be overwritten manually by
means of program keys, provided that the door is open.
The program will launch only after door is completely closed.
Display first shows program name, subsequently status indication as described here:.
P2 W●●_ _ X13 Min
Cleaning 36 °C
P2 Program N° (P1 – P12)
W●_ _ XProgram step status, e.g. step 1 of 3
W●●_ _ XStatus indication, step 2 of 4.
End marking "W_ _ X"represents the number of steps in the respective program.
13 Min Remaining run time or remaining program time.
Actual value plus recorded last value / 2. In case of interruption, value is ignored.
Cleaning Current step
36 °C Temperature of wash liquid or of drying air at input wash channel.
3.3 User Identification
Importing Names – N°. by way of the keyboard or importing Name of user by means of the code reader for
the batch documentation.
With active user ID, and with open door, the following message, i.e. interrogation is found on display:
User Name or N°?
--
The unit either reads a 2-digit of the 6 program keys from 11 - 66 or a text / number by means of the barcode
reader. The respective number (e.g. 36) or name is passed on to the printer or the digital documentation sys-
tem, it is also associated with the respective batch. A program can be launched only after the respective in-
puts are complete, otherwise the SW persists with the interrogation.
Input by keyboard:
IN case of input of N° 66, text „No ID “ is passed on.
IN case of input by barcode reader, it is preferable to establish a list with N° associations with respective
names.
Example:
N° Name
11 Sr. A. Muster
12 Sr. H. Mueller
66 No ID

Operating Manual WD 425E
Rev. 1.1, LS Page 17 of 34 78563_E rev 1.1.doc
Input by barcode reader:
When entering N° 0, text „No ID“ is passed on.
Barcode Type: EAN 13, connection of the machine with 8535BC.
For internally used machines:
Barcode Type: Code 39. There is a length restriction of 20 characters per name. It is easy to produce name
tags in house using simple programs. They may be downloaded from the internet at no cost, see under
http://www.barcodemagic.com/barcodemagic.html.
Barcode Type Code 39 EAN13
Heike Müller
Heike Mueller No ID / Exit
The interrogation may be activated / deactivated in the configuration module.
3.4 Rack Detection
Importing rack or Wash goods carrier-N° by keyboard or importing rack name by barcode reader for
batch documentation.
With active Rack detection, the following is displayed:
Rack name or N°?
--
The machine either reads a 2-digit number of the 6 program keys from 11 - 66 or a text by barcode scanner.
The respective number (e.g. 36) or name is passed on to the printer or to the digital documentation system
8565 and assigned to the respective batch. A program can be launched only if the input is complete, other-
wise the SW remains in interrogation mode.
Input by keyboard:
Input N° 66 is passed on as text „No ID “.
Input by barcode reader:
Importing N° 0 will be passed on as „N° ID “.
Barcode Type Code 39, the name is limited to 20 characters.
Interrogation may activated / deactivated in the configuration module 5.
3.5 Identification Batch Content
Importing N° of wash goods (e.g. Sieve trays) by keyboard or importing wash goods name or N° by barcode
reader for batch documentation.
With active Batch ID and open door, instead of „UC door open “, the following is displayed:
Read Batch Content
N° = -- ?
The machine either reads a 2-digit number of the 6 program keys from 11 - 66 or a text or a N° by barcode
scanner. The respective number (e.g. 36) or name is passed on to the printer or to the digital documentation
system 8565 and assigned to the respective batch.

Operating Manual WD 425E
Rev. 1.1, LS Page 18 of 34 78563_E rev 1.1.doc
As a maximum, 18 objects may be imported. The input must always be completed by N° 0. It is not possible
to enter the same number or name twice. A program can be launched only once the input is complete, oth-
erwise the SW remains in interrogation mode.
Input by keyboard:
Entering N° 66, text „No ID“ is passed on and the procedure is terminated.
Input by barcode reader:
If no object needs to be entered, e.g. OP shoes, inputting N° 0 will terminate the procedure. Text „No ID“
will be passed on.
The reading process may be terminated at any time by the „IO“ key. All inputted data will be erased.
At he end, always conclude with N° 0. On the display, indication of number of batch contents terminate the
procedure.
Once the maximum of 18 batch contents is reached, following inputting the 18th item, instead of „Read batch
content “, „Max batch content “ appears and the importing will be terminated without entering N° 0, i.e. the
program may be launched.
N° Batch Content
12
The rack may be pushed in machine.
Barcode Type EAN 13. The length of the name is limited to 12 characters.
Preferably, the barcode N° 0 is attached to the unit.
No ID / Exit
Interrogation may be activated / deactivated in configuration module 5.

Operating Manual WD 425E
Rev. 1.1, LS Page 19 of 34 78563_E rev 1.1.doc
3.6 Endoscope Identification
With active endoscope detection, with door open, display shows “Interrogation Batch Content” instead of
„Door open “. With door button, leak test may be started simultaneously:
Read Content N° / Input batch content
Endo L = - - ?
With barcode reader or keyboard, read batch content left hand endoscope or keyboard.
SW reads a 2-digit number (12 CHAR) with 12 characters , e.g. 000000000012.
Read Content N° / Read batch content
Endo L= 12
The number read (e.g. 12) or the name is passed on to the printer RS232 X 31 or to the interface RS 485 X32 to
show on display.
As soon as the first number is input, the right hand endoscope is interrogated. Display shows:
Read Content No. / Read batch content
N° R = - - ?
If no endoscope is placed in spot, by N° 66, the next interrogation will be pursued or reading of right hand endo-
scope may be terminated.
Read Content No. / Read batch content
N° R = No identification
Instead of N° 66, printer shows “No ID”
Following reading in the second endoscope, display shows
Program ready
Door open
If there is no input and a program is started, display shows „No ID “.
If unintentionally an incorrect number is entered, pushing the I/O button will disrupt the entire procedure. All previ-
ously entered data will thus be lost.
Program cut off
13.02.2003 11.15
With active endoscope ID, printer will show the line „Batch content.

Operating Manual WD 425E
Rev. 1.1, LS Page 20 of 34 78563_E rev 1.1.doc
4. Program Launch and Process Sequence
!IMPORTANT NOTE!
• Periodic instruction sessions for users is designed to assure cleaning quality.
• Avoid prolonged decontamination film drying time of medical products.
• Prior to machine type processing, drive elements for drill bits and milling tools or complex
medical products need to be disassembled. They must be pre-treated by mean of ultra sound or
manual brushing. Follow the respective manufacturer’s instructions.
• Hollow bodies for drive elements or MIC instruments generally need connection of hoses at the
wash good racks. This in order to assure decontamination and disinfection of inner surfaces of
the respective medical products.
1. Check liquid level of detergent. If needed, replenish container.
2. Open supply shut off valve and energize machine by main switch.
3. Push 0-I switch. Display shows „Program ready “
4. Press door key to open wash chamber door, remove insert rack.
5. Load appropriate rack only with wash goods. Be sure that water is always capable of running
of wash goods and that all wash arms cane freely rotate.
6. Drain all containers, remove plugs, corks, tags, sealing wax remnants etc. Wash good utensils may
not be piled inside each other and thus cover each other.
7. The wash arms must not be blocked by protruding (top or bottom) wash good elements. Carry
out manual rotation check.
8. Wash goods must not stick out of the racks. When pushing rack into machine, protruding sharp ob-
jects might otherwise be harmed.
9. When pushing rack into machine, it is mandatory that water docking elements match those of
the machine!
10. !DANGER!
A fully loaded rack may weigh up to 100 kg. Caution, a falling rack may put operators at risk of
injury.
11. With active batch ID, the barcode reader may identify the user, the wash goods rack and also the
batch content (endo basket, sieve trays).
12. Fully insert wash goods rack until docking elements for supply are felt snapping into their counter-
parts of the machine.
13. With the WD 425, a clicking sounds must be audible and display must show „Program ready “.
14. The machine is now ready for launch.
15. Press the desired program key. Check if water is received by the wash chamber. Display initially
shows the program name, subsequently the respective program status.
16. During the program run, the display will keep you informed about the current program step, the cur-
rent temperature inside the wash chamber and about the remaining program run time.
17. During the entire wash cycle, the wash chamber door remains locked.
18. If during the program cycle the program is stopped by means of the I/O button, the door remains
locked nevertheless . Instead of program, the display shows „Door locked „. The door can be unlo-
cked only at the end of a full and successful program cycle. The door also remains locked as long as
the level sensor shows >0.5V (water in machine).
Table of contents
Other BELIMED Washer manuals