BioMedical Life Systems, Inc. Impulse TENSD5 User manual
34
12
Patient Safety Information
Caution
Federal law (USA) restricts this device to sale by or on the
order of a physician so licensed by the State.
Indications
Transcutaneous Electrical Nerve Stimulation (TENS) devices
are used for the symptomatic relief and management of
chronic (long-
term) intractable pain and as an adjunctive treat-
ment in the managem
ent of post-surgical and post-traumatic
acute pain problems.
Contraindications
TENS devices can adversely affect the operation of demand-
type cardiac pacemakers. TENS is not recommended for
patients with known heart disease without a physician’s
evaluation of risk. Do not stimulate over the eyes or carotid
sinus nerves. Do not apply TENS for undiagnosed pain syn-
dromes until etiology is established. Do not place electrodes
in a manner that causes current to ow transcerebrally
(through the head).
Warnings
This device should be used only under the continued su-
pervision of a physician, or outside the USA, by a qualied
pain management specialist. TENS is ineffective for pain of
central origin. TENS is of no curative value; it is a symptom-
atic treatment which suppresses pain sensation which would
otherwise serve as a protective mechanism on the outcome
of the clinical process. Safety of TENS devices for use during
pregnancy or delivery has not been established.
Electronic equipment such as ECG monitors and
ECG alarms
may not operate properly when TENS is in use. Using
this
device in proximity to any object that produces an elec-
tromagnetic current such as a microwave oven or cellular
telephone
could affect the performance of the device. The
Operating Instructions
user must keep the
device out of the reach of children. TENS
is for external use only.
Use of electrodes and accessories
Electrodes used with the device should be no smaller than
3
/
4
" in
diameter. Please note that the smaller the size of the
electrode used,
the greater the intensity of stimulation at the
electrode site which increases the likelihood of skin irritation
at the site. Only BioMedical Life Systems authorized electrodes
and accessories are to be used with this device. If you have
any questions, please contact
either your dealer/distributor
or BioMedical Life Systems directly.
Precautions
Avoid adjusting controls while operating machinery or ve-
hicles. Turn the stimulator off before applying or removing
electrodes. Isolated cases of skin irritation may occur at the
site of electrode placement following long-term application.
Use only for the specic pain problem as
prescribed by the
physician, or outside the USA, by a qualied pain management
specialist. Effectiveness is dependent
upon patient selection
by a qualied pain specialist.
EQUIPMENT not suitable for use in the presence of a FLAM-
MABLE ANESTHETIC MIXTURE WITH AIR OR WITH OXYGEN
OR NITROUS OXIDE
Adverse Reactions
Possible allergic reaction to tape or gel. Possible skin irrita-
tion or electrode burn.
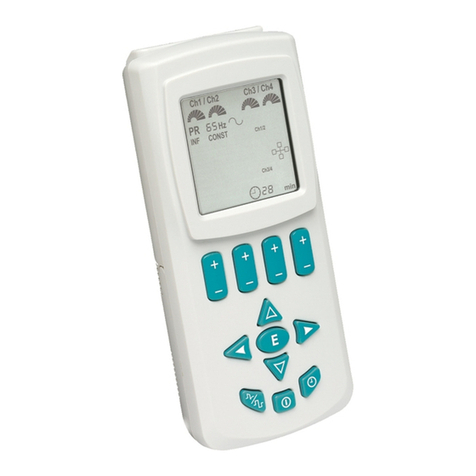
This device is a Transcutaneous Electrical Nerve Stimulator.
One pair of electrodes can be connected to each output
channel using the leadwires supplied. Stimulation pulses
are transferred from the device through the lead
wires to the
electrodes. The intensity, duration, and number
of pulses per
second can be adjusted.
Instructions for use
• Attach leadwires to Channel 1 (CH1) and, if instructed
by clinician, to Channel 2 (CH2). (1 and 2)
• Attach electrodes to leadwires following instructions on
electrode packaging.
• Place electrodes on body as directed by clinician.
• Turn on device (9).
• Readout similar to (3) will appear on Display Screen.
• If no electrodes are applied to the body, a safety
feature is enabled and the amplitude drops to
zero and an "open electrode" symbol ashes on
the screen.
Programming a Stimulation Pattern
Select the desired stimulation pattern by pushing the Mode
Button (6) until the desired stimulation pattern is displayed
on the Screen (3). The patterns will appear in the following
sequence:
CONSTANT = Constant/Continuous
BURST I = BURST I
BURST II = BURST II
PW. MOD. = Pulse Width Modulation
PR. MOD. = Pulse Rate Modulation
Select the desired pre-programmed stimulation pattern by
pushing the Mode Button (6) until the desired patten is
displayed on the screen (3).
Accessories
2 Leadwires with elec-
trodes
Only use accessories, electrodes,
leadwires and batteries approved
by BioMedical Life Systems, Inc.
We do not recommend the use of
rechargeable batteries, as they may
weaken the performance and/or
read-out of the device.
1 Instruction Booklet
2 Batteries
1 Carry Pouch
Technical Data
Dimensions 4.2” x 2.9” x 1.4”( 10.7cm x 7.4cm x 4cm)
Weight 3.35 oz (94.97 grams)
Channels Dual
Power Sup-
ply
2 AA Batteries, Type LR6
Waveform Symmetrical, biphasic square wave
Pulse Rate
(Hz)
1 - 120 Hz (Hertz or pps) adjustable
Pulse Width
(µS)
25 - 250 microseconds (μs) adjustable
Constant Continuous stimulation. Pulse Rate/Pulse
Width are adjustable.
Pulse Rate
Modulation
Pulse Rate modulates from 100Hz down to
20Hz over a 15 second cycle (7.5 seconds
down 7.5 seconds up. Pulse width is adjust-
able.
Pulse Width
Modulation
Pulse width modulates from 125-250μS
and back down over a 5 second cycle (2.5
seconds down, 2.5 seconds up). Pulse Rate
is adjustable.
Burst I One second ON. One second OFF. Pulse Rate
and Pulse Width are adjustable.
Burst II
Seven Pulses per burst, 2 bursts per second.
Pulse Width is adjustable.
Output Constant current
Intensity Continuously adjustable from
0- 100 mA peak to peak
Output Volt-
age
Continuously adjustable from 0-50 V peak
to peak
Tolerances +/- 1%
(Data was recorded across a 500 OHM resistance load.)
Figure A
CONST
Figure B
PW MOD
Figure C
PR MOD
Figure D
BURST I & II
Graphic Symbol Denitions
mA CH1 mA CH2
TIMER MODE ON/OFF
P.W. (µS) P.R. (Hz)
+
_+
_
+
_+
_
Impulse®
TENSD5
1
2
11
10 7 6 8
9
45
3
CONSTANT
120 Hz
50 µs
20
15
10
5
20
15
10
5
Ch1 Ch2
Refer to oper-
ating instruc-
tions
An IEC 601-1 safety
standard
(type BF)
0086
We herewith declare that the
above mentioned product meets
the provisions of the Medical De-
vice Directive
Figure 1
All pre-programmed stimulation patterns can be pro-
grammed for individual needs:
Increase or decrease the P.R. value by increasing the (+)
or decreasing the (-) P.R. button (8). The P.R. is adjustable
from 1-120 Hz in 5 Hz increments. Increase or decrease the
P.W. value by increasing the (+) or decreasing (-) P.W. but-
ton (7). The P.W is adjustable from 25-250 μS.
• Select the desired treatment time by pressing the Timer
Button (10) until the desired treatment time is displayed;
Continuous, Adjustable 10, 15, 20, 30, 45, 60 minutes
When a treatment time has been selected, the device
will count down the elapsed time and automatically turn
off.
• Increase or decrease the intensity of the device by press-
ing down the +/– for CH1 (4) or CH2 (5). There are 20
levels of intensity available.
• If a new mode selection (6) is made during treatment,
the intensity of the device automatically drops to Level
0.
• To immediately turn off the device at any time press the
On/Off (9) button.
• After the treatment period, disconnect leadwires from
device (1 & 2). Store electrodes as per instructions on
electrode package. If the device is not going to be used
for long periods of time the batteries should be removed
(11).
Programming
7
56
Copyright © 2006 BioMedical Life Systems, Inc.
All Rights Reserved
Instructions
Safety and Technical Checks
Once a year, a maintenance check should be performed on
the device as follows:
• Visually check the exterior case of the device for dam-
age.
• Visually check the input and output sockets for dam-
age.
• Visually check the device for clarity of reading instructions
and indicator decals.
• Visually check that the illumination of the LCD is operat-
ing correctly.
• Visually check the leadwires and electrodes for wear.
Should any malfunctions occur while using this device,
check:
• Whether the leadwires and electrodes are correctly con-
nected to the device. The leadwires should be inserted
rmly into the device sockets.
• Whether the screen (LCD) is illuminated. If not, insert
new batteries.
• For possible damage to the leadwires. Change the lead-
wires if any damage is detected.
Do not attempt to repair a device yourself!
Opening the device case voids the warranty. Please contact
the dealer from whom the device was purchased. If they are
unable to assist you, please contact:
In the USA and Canada, BioMedical Life Systems, Inc., (760)
727-5600.
In Europe, BMLS BV, Alkmaar, The Netherlands.
T
his device MUST only be serviced by the manufacturer.
To reorder any accessories or supplies, contact your dealer.
Warranty
LIMITED WARRANTY (USA only, unless otherwise
noted)*
BioMedical Life Systems, Inc. promises to the original con-
sumer-purchaser to repair or, at the option of BioMedical Life
Systems, Inc., to replace any neurostimulator which malfunc-
tions or proves defective in materials or workmanship under
normal use during the period of the Warranty. During this
time, BioMedical Life Systems, Inc. will provide all labor and
parts necessary to correct such defects or malfunctions free of
charge. If the product is no longer available, BioMedical Life
Systems, Inc. reserves the right to substitute a comparable
product. The consumer-purchaser is responsible for all ship-
ping charges when returning the device to the manufacturer
or designated service facility.
EXCLUSIONS
This warranty shall not apply to damage resulting from failure
to follow
these Instructions, accident, abuse, alteration, or
disassembly by unauthorized personnel. This warranty does
not extend to accessory items such as rechargeable batteries,
electrodes, leadwires, and conductive gel. These items can
be provided by your dealer, but costs for repair or replace-
ment will be the responsibility of the consumer-purchaser.
BioMedical Life Systems, Inc. shall not be liable for incidental
or consequential damages resulting from the sale or use of
the device. In the USA, some states do not allow the exclu-
sion or limitation of incidental or consequential damages, or
do not allow limits on how long an implied warranty lasts,
so the above limitation may not apply to you.
NO OTHER WARRANTIES
This limited warranty is the only express warranty given by
BioMedical Life Systems, Inc. Implied warranties, including,
but not limited to, warranties of merchantability and tness
for a particular purpose are limited to the warranty period set
forth below. This warranty gives you specic legal rights, and
you may also have rights which vary from state to state.
If the device case is opened or tampered with in any way,
all warranty coverage is void.
* In the USA, unless otherwise indicated, the limited War-
ranty is three years. Outside the USA, please check with
your distributor to ascertain the “Limited Warranty Period.”
IMPULSE TENS D5 ENGLISH 04/22/2006 REV.NEW
Transcutaneous Electrical Nerve Stimulator
Impulse
®
TENSD5
BioMedical Life Systems,Inc.
Malfunctions
Maintenance and Care
Tips for Skin Care
Skin should be cleaned prior to placement of the electrodes. If
the electrodes do not contain gel, then gel should be applied
directly to the skin prior to placement of the electrodes.
Electrode Placement Alternatives
• Place directly over the area from which the pain is ema-
nating.
• Encircle the area of pain.
• Place proximally above the main nerve stem of the pe-
ripheral nerve responsible for the pain area.
• On specic points such as trigger points or acupuncture
points.
• Place in the area of the pain site.
The treatment, when applied independently or in conjunction
with medicinal therapy, should rst be attempted with Low
Frequency TENS treatment control settings.
A consistent application of approximately 2 Hz has been
shown to produce effective stimulation.
The Amplitude and Width settings should be set as high as
possible without causing discomfort. The treatment period
should be at least 20 - 30 minutes as the pain-inhibiting ef-
fect only commences after approximately 15 - 20 minutes.
In the most favorable case, treatment lasting thirty minutes
could contribute to a reduction in the need for analgesics.
This will, however, be dependent upon the seriousness of
the patient’s condition.
Should Low Frequency TENS treatment not yield the desired
result, High Frequency TENS treatment should be applied
as follows:
(High Frequency TENS Treatment) Frequencies are found in
• The case housing is made of insulated ABS plastic and
can be cleaned with isopropyl alcohol.
• Stubborn stains and spots can be removed with a clean-
ing agent. Do not submerge this device in any liquid or
use excessive cleaning liquid when cleaning the surface
area.
• NOTE: Do not smoke or work with an open ame (for
example, candles, etc.) when working with ammable
liquids!
the range of 100 - 150 Hz. The pulse width settings are gen-
erally set between 10 - 100 μs. However, the wide range of
settings on this device allows the treatment to be customized
to achieve optimal results for the patient.
The pain-inhibiting effect should commence within a few
minutes. The treatment period should be between 20 - 30
minutes. In some cases, desensitizing must be carried out
for several applications.
The correct level of stimulation should feel comfortable to
the patient and should never be set at levels that cause
discomfort.
Warning:
Only electrodes and leadwires authorized by the
device manufacturer should be used.
In order to maintain the functional operation of the
Impulse®TENSD5 the batteries will have to be changed periodi-
cally. The device is supplied with 2 AA Alkaline batteries.
Warning: We do not recommend the use of rechargeable
batteries, as they may weaken the performance and/or
read-out of the device.
To change batteries:
• Before opening the battery compartment, check to make
sure that the device is switched off (9).
• Slide the battery compartment cover (11) down.
• Remove the batteries (11) from the compartment. Gently
insert the new batteries by matching the +/– end of each
battery with the +/– symbol found inside the battery
compartment.
• Replace the battery compartment cover and slide up to
close.
• Remove the batteries if you do not plan to use the device
for long periods of time. Otherwise leakage and damage
to the device can occur.
• Dispose of batteries in a proper manner.
BioMedical Life Systems, Inc.
2448 Cades Way
Vista, California 92081-7830,
USA
Tel: (1) (760) 727-5600
BioMedical Life Systems, BV
Postbus 6
1800 AA Alkmaar,
Netherlands
Batteries
Recommendations for the Therapist
Impulse®
TENSD5
ENGLISH
Table of contents
Other BioMedical Life Systems, Inc. Fitness Equipment manuals