bioMetric SpinalPak User manual

Biomet®SpinalPak®
Non-invasive Spine Fusion
Stimulator System
Complete Manual
and Package Insert

1
Table of Contents
SYSTEM CONTENTS.....................................................................................................................1
IMPORTANT SAFEGUARDS .........................................................................................................2
BIOMET®SPINALPAK®NON-INVASIVE SPINE FUSION STIMULATOR SYSTEM .........................3
• Description............................................................................................................................3
• ElectricalRequirementsforBatteryPackandCharger .........................................................3
SYSTEM COMPONENTS ............................................................................................................3,4
FULL PRESCRIBING INFORMATION.............................................................................................5
• IndicationsforUse................................................................................................................5
• Warnings,Precautions,AdverseEffects ...............................................................................5
DIRECTIONS FOR USE..................................................................................................................6
• RecommendedUsage...........................................................................................................6
OPERATING INSTRUCTIONS.....................................................................................................6,7
CHARGING THE BATTERY PACK...............................................................................................7,8
BUTTON FUNCTIONS....................................................................................................................9
LCD SYMBOL DESCRIPTION AND INSTRUCTIONS .....................................................................9
TREATMENT COMPLETION ........................................................................................................10
PATIENT COMPLIANCE MONITORING ......................................................................................10
ORDERING INFORMATION .........................................................................................................10
ELECTRODE INSTRUCTIONS FOR USE ......................................................................................10
SYMBOL DESCRIPTION..............................................................................................................11
EQUIPMENT CLASSIFICATION ...................................................................................................11
CLEANING INSTRUCTIONS ........................................................................................................11
ELECTROMAGNETIC COMPATIBILITY.........................................................................12,13,14,15
PATIENT COUNSELING INFORMATION......................................................................................16
STORAGE AND HANDLING ........................................................................................................16
DISPOSAL INSTRUCTIONS .......................................................................................................16

1
System Contents
• Electrodes-SoftTouch®- 72R
• Electrodes-SoftTouch®- LT-4500
• ChargerCradle
• RechargeableBatteryPacks(2)
• ElectrodeCovers
• Stimulator
• DeviceHolster
• LeadWires-20”LeadWireand48”LeadWire
• PatientManual
• CompleteManualandPackageInsert
• A/CAdaptor

2 3
Important Safeguards
READ ALL INSTRUCTIONS BEFORE USING
SAVE THESE INSTRUCTIONS
Whenusingelectricalproducts,basicsafetyprecautionsshouldalwaysbefollowed,including:
ATTENTION: To reduce the risk of electric shock, fire or injury:
1. Donotusethisproductwhilebathing,showeringorswimming.
2. Donotplaceorstorethisproductwhereitcanfallorbepulledintoatuborsink.
3. Donotimmersethestimulator,batterychargerorthebatteryinwateroranyliquid.
4. Donotreachforthisproductifithasfallenintowater.Unplugfromthewalloutletimmediately.
5. Donotpermitthebatterychargertobeconnectedwhenwet.
6. Donottouchthebatterycontactswhenthebatterychargerispluggedintoanoutlet.
7. Neveroperatethebatterychargerifithasadamagedpowercord,plugorifitisnotworking
properly.Donotuseifithasbeendroppedanddamaged,orimmersedintowater.Contact
Biometforreturninstructions.
8. DonotattempttochargeanyothertypeofbatterypackintheBiomet®SpinalPak®Non-invasive
SpineFusionStimulatorSystembatterycharger.
9. Keepallcordsawayfromheatedsurfaces
10. Neverinsertanyforeignobjectintoanyopeningofthesystem.
11. Donotexposethestimulatororthebatterychargertoprolongedheatordirectsunlight.
(Normaloperatingtemperaturerangeis5°Cto38°C[41°Fto100°F],normalstorage/transport
temperatureis-15°Cto50°C[5°Fto122°F].
12. Usethisproductonlyforitsintendeduseasdescribedinthismanual.
13. TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemhasnoinstallation,
periodicmaintenancerequirementsoruserserviceableparts.Ifanyofthereplacementpartsare
damagedtheymustbereplacedbyBiometinordertoavoidahazard.
14. Donotshortcircuit,overcharge,crush,mutilate,nailpenetrate,heat,reversethe+or-
terminalsordisassemblethebatterypack.Donotallowmetalobjectstocomeintocontactwith
thebatteryterminals.Theseandanyotherabusesofthebatterypackmaycauseseriousinjury
and/orburns.Toensureproperchargingandsafety,useonlythechargersuppliedwithyour
device.Keepthebatterypackdry.Thisbatterypackmustbedisposedofproperly.Disposal
informationcanbeobtainedbycontactingtheRechargeableBatteryRecyclingCorporation
(RBRC)at1-800-822-8837intheUS.
NOTE: Inside the United States call Biomet at 1-800-526-2579 or 1-973-299-9300 if calling from
outside the United States with any questions or problems.

2 3
Biomet®SpinalPak®Non-invasive Spine Fusion Stimulator System
Caution: Federal Law (U.S.A.) restricts this device to sale by or on the order of a physician.
Rx Only.
2
Thisdeviceisnotintendedforre-saleorre-distribution.Singlepatientuse.
DESCRIPTION
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystem (Figure1)promoteshealing
byinducingalowelectricalcurrentatthefusionsite.Thetherapeuticsignalgeneratesalowenergy
electricalfieldbypassingaspecificcurrentbetweentheelectrodes.
ELECTRICAL REQUIREMENTS FOR BATTERY AND CHARGER
Charger
Input:100-240VAC50/60Hz6W
Output:12VDC500mA
ForusewiththeBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystembatterypack
only(PN1067720).
Batteryrating:3.7VDC>800mAh
Donotusethebatterypacksuppliedwiththisunitinanyotherdevice.UseoftheBiomet®SpinalPak®
Non-invasiveSpineFusionStimulatorSystembatterypackinanyotherdevicemaycausedamageor
malfunctiontothebatterypackand/ordevice.
System Components
STIMULATOR
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemoperatesonarechargeablebatterypack,
whichallowsforambulatoryuse.Itincludesanaudibleandvisibleself-checkingalarmmechanismtoalertthe
patientifitisnotfunctioningproperly.TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystem
isdesignedtostorethepatient’sdailytherapeutictreatmentdatawhichmaybedownloadedandreadwiththe
patientcompliancesoftware(SeePatientComplianceMonitoringPage10).Patientsareencouragedtobringthe
stimulatortoeachfollow-upvisitwiththeprescribingphysiciantoreviewhowtheyareusingtheirstimulator.
Figure 1

4 5
System Components
BATTERY PACK AND BATTERY CHARGER
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemincludestworechargeablebatterypacks.
Uponreceipt,itisrecommendedthatthesecondbatterypackbeimmediatelyplacedintothechargercradleandfully
charged.Inthemeantimethefirstbatterypackmaybeusedtobeginyourtreatment.
Note: The first battery pack may not provide a 24-hour treatment initially. It is recommended that the patient keep one
battery pack in the battery charger cradle to assure that it is fully charged and ready, and the other inserted into the
Biomet®SpinalPak®Non-invasive Spine Fusion Stimulator System. This will ensure continuous treatment prescribed by
the prescribing physician.
ThebatterychargerandcradlearedesignedtorechargetheBiomet®SpinalPak®Non-invasiveSpineFusionStimulator
Systembatterypacksonly.TwoLED(lightemittingdiode)lightsmonitorandindicatetheoperationalstatusofthebattery
charger.TheA/CPowerindicatorlightislocatedontheA/CAdaptor.Thechargingstatusindicatorlightislocatedonthe
chargercradle.ThefollowingtablelistsanddescribestheoperationalfunctionsoftheLEDlights:
Electrodes
TherearetwotypesofelectrodesthatarepackagedwiththeBiomet®SpinalPak®Non-invasive Spine
FusionStimulatorSystemassembly:72RandLT-4500.The72Relectrodeshavegreenwritingontheir
packaging.TheLT-4500electrodeshaveblackwritingontheirpackaging.The72Relectrodeshavea
hydrogelthatisstickierthantheLT-4500electrodehydrogel.Thepatientcanusewhicheverelectrodes
bestsuittheirskintype.
Electrode Covers
Theelectrodecoversarewaterresistantandareintendedtoenhanceelectrodesecuritytotheskin,if
needed,orforshoweringwiththeelectrodesattached,ifdesired.
Device Holster
Thedeviceholster isdesignedtosecurely holdtheBiomet® SpinalPak®Non-invasiveSpine Fusion
StimulatorSysteminplace.Ithasacliponthebackwhichallowsthepatienttowearthedeviceon
theirwaistbandorbelt.
Lead Wires
TwodifferentlengthleadwiresareincludedwiththeBiomet®SpinalPak®Non-invasiveSpineFusion
StimulatorSystem.Thepatientshouldchoosetheleadwirethatbestaccommodatestheirneedsfor
wheretheywouldliketowearthecontrolunit.
Following are possible error conditions and possible resolutions.
Status A/C Power
Indicator Light
Charging Status Indicator
Light on Cradle
Nobatterypackinserted
(idle)onA/Cpowered
batterycharger
Solidgreen Off
Batterypackinchargingstate Solidgreen Solidorange
Fullychargedbatterypack Solidgreen Solidgreen
A/Cpowerdeficiency Off Off
Error Solidgreen Off
Error Conditions (flashes orange) Possible Resolutions
Batterypacknotproperlyconnected
tothecharger
Removeandre-installthebatterypackto
ensureacompleteconnectiontothecharger
Batterytemperatureistooloworhigh Normaloperatingtemperatureis
5°Cto38°C[41°F-100°F]
Batteryvoltageistoolow CallBiometforanewbatterypack

4 5
Full Prescribing Information
INDICATIONS FOR USE
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemisanoninvasivespinefusion
stimulatorindicatedasanadjunctelectricaltreatmenttoprimarylumbarspinalfusionsurgeryforone
ortwolevels.
WARNINGS
Cardiac pacemakers or cardioverters may be adversely affected by the Biomet® SpinalPak®
Non-invasiveSpineFusionStimulatorSystem.Theconcomitantuseofthedeviceandapacemakeror
cardiovertermustbeassessedonanindividualbasis,suchaswithanelectrocardiogram,priortouse.
Thepatientshouldbereferredtoacardiologistformonitoringofpacemakerfunctionwhilewearing
theactiveBiomet®SpinalPak® Non-invasiveSpineFusionStimulatorSystemdevice.Ifthereareany
observableadversechangesinthepacemakerrhythmoroutput,thedeviceshouldnotbeused.
ThesafetyandeffectivenessoftheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystem
inpregnantwomenhavenotbeenstudied,andtheeffectsofthedeviceonthemotherorthedeveloping
fetusareunknown.Apatientwhoiseitherpregnantorisintendingtobecomepregnantshouldbe
referredtoherdoctorpriortotreatmentwiththedevice.
PRECAUTIONS
ThesafetyandeffectivenessoftheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystem
in individuals with the following conditions have not been studied, and therefore the safety and
effectivenessofthedeviceintheseindividualsareunknown:
– spondylitis,infection,Paget’sdisease
– cancer,diabetesmellitus,renaldisease
– traumaofthelumbarspine
– osteoporosis.
Applytheelectrodesaftertheskinhasbeencleanedanddried.Iferythemadevelopsattheelectrode
sites, the electrodes should be relocated adjacent to the original sites. If the reaction does not
resolveafter48hoursafterrelocatingtheelectrodes,thepatientshouldbeinstructedtoconsultwith
thephysician.
Donot submergeorexpose theBiomet® SpinalPak®Non-invasiveSpine FusionStimulatorSystem
to water. The patient must be instructed to remove the stimulator during bathing, showering
orswimming.
Compliance with the treatment schedule, daily battery pack changes, and replacing the electrodes
(1to7days)asneededareessentialforproperdevicefunction.Thissystemshouldonlybeusedwith
componentsandreplacementpartssuppliedbyBiomet.Othercomponents,partsandaccessoriesmay
notbecompatible,andmaydamagethedevice.Ifanycomponentdoesnotfunctionproperly,contact
Biomet.Noattemptshouldbemadetomodifyorrepairthedevice.
Patientsshouldbeabletousethedeviceinaccordancewiththeinstructionsforuse.If a patient cannot
comply with these instructions for any reason, use of the device is not recommended.
ADVERSE EVENTS
Duringamulti-centerclinicalstudy of349patients treatedwiththe device fortheindication listed
above,skinirritationwasthemostcommonadverseeffectassociatedwiththeuseofthedevice.It
occurredin9patients(2.6%ofthetrialpopulation):4patientstreatedwiththeactivedeviceand5
patientstreatedwiththeplacebodevice.

6 7
Directions for Use
RECOMMENDED USAGE
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System is designed to deliver 270
daysofcontinuoustherapeutictreatmentfor24hoursperday.Therecommendeddailytherapeutic
treatmentiscontinuousfor24hours.
OPERATING INSTRUCTIONS
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemhasbeenspecificallydesigned
tobeconvenienttouse,comfortabletowear,andsafetooperate.Patientsshouldbeginusingthe
Biomet® SpinalPak®Non-invasive Spine Fusion Stimulator System immediately after reading the
instructionsforuseandhavingreceivedinstructionsfromtheirprescribingphysician.
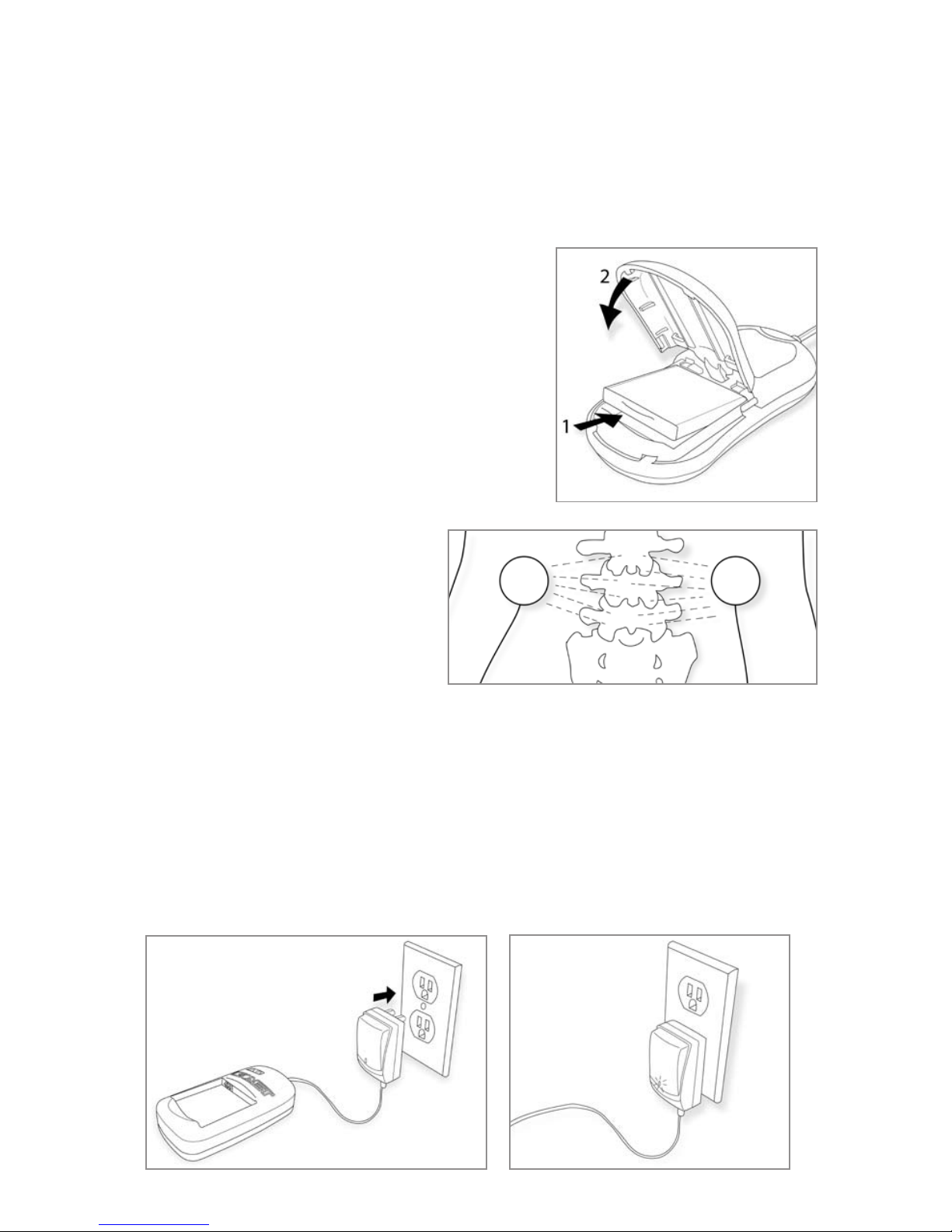
To begin treatment - Electrode Placement
• Cleananddrytheskinwheretheelectrodeswillbeplaced.Trimming(notshaving)bodyhairfrom
theelectrodeapplicationareaisoftenhelpful.
• Note: To ensure electrode placement and adhesion, you may use one of the provided electrode
retainer patches. Place one electrode
twoto threeinchesto theleft ofthe
area of the surgery and a second
electrode two to three inches to the
rightoftheareaofthesurgerysothat
the electrodes are four to six inches
apart. See Figure 2.Dependingonthe
patient’sabilitytomoveaftersurgery,
it may be helpful for the patient to
ask another person to assist them
inplacing the electrodes.See instructions foruse on Page10. The patientshould consult their
prescribingphysicianorBiometiftheyhaveanyquestionsorconcernsregardingproperelectrode
placement.Iftheirskinbecomesabnormallyredattheelectrode sites,theelectrodesshouldbe
moved adjacent to the original sites. If the redness does not go away after 48 hours with the
electrodesremoved,thepatientshouldcontacttheirprescribingphysician.
Tips:
Loose electrodes–Confirmthatbothelectrodesareincompletecontactwithclean,dryskin.See
instructionsontheelectrodepacket.
Incomplete circuit/disconnection –Checkallconnectionpoints,confirmingasnugfit.
Broken electrode lead wire–Ifalarmingcontinues
afterconfirmingconnection,attachanewelectrode
leadwire.
To begin treatment - Lead Wires
• Thepatientisprovidedwithtwostimulatorleadwire
lengthswiththeBiomet® SpinalPak®Non-invasive
SpineFusionStimulatorSystem.Thepatientshould
selectthelengthofthestimulatorleadwireinorder
to provide both convenience and comfort when
wearingthestimulatorduringtreatment.
• Insertthestimulatorleadwiremaleconnectioninto
eachfemaleelectrodeleadwireconnection.
• Inserttheleadwireplugintotheopeningatthetopofthestimulator.See Figure 3.
Figure 2
Figure 3
6 7
Operating Instructions
Both battery packs provided with the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator
Systemarechargedpriortobeingpackagedanddistributed.UponreceiptoftheBiomet®SpinalPak®
Non-invasive Spine Fusion Stimulator System, it is recommended that the second battery pack be
immediatelyplacedintothechargercradleandcompletelycharged.Inthemeantime,thefirstbattery
packmaybeusedtobeginyourtreatmentimmediately.Note: The first battery pack may not provide
a complete 24-hour treatment initially.
STEP 1:
Following the arrows, insert the charged battery pack
(1,2)intotheBiomet®SpinalPak®Non-invasiveSpineFusion
Stimulator System (Figure 4). The LED light on top of the
device will blink, indicating power. Each symbol will be
indicatedonthedisplayandthealarmwillflashandbeepif
theelectrodesarenotproperlyapplied.Tosilencetheaudio
alarmpressthebuttonbelowthedisplay.
If the light does not blink, which indicates that the battery
packisnotcharged,changethebatterypack
(SeeChargingtheBatteryPackbelow).
STEP 2:
Attach electrodes as per instructions on
Page10, and asper “To BeginTreatment -
ElectrodePlacement”sectiononpage6.
Charging the Battery Pack
Chargethebatterypackatroomtemperature(24°C(75°F)).Chargingmayrequiretwotothreehours.
Chargingmayvaryinwarmerorcoldertemperatures.
STEP 1:
Plugthebatterypackchargerandcradleintoawalloutlet(Figure5).AgreenlightontheA/Cadaptor
willilluminateindicatingpower(Figure6).
Figure 4
Figure 5 Figure 6

8 9
STEP 2: CHANGING BATTERY PACKS
Eachday,preferablyatthesametimetoensuretreatment
is continued without interruption, patients should do
thefollowing:
A. Depressthelatch(1)onthebatterydoorandslide
downthebatterydooronthebackofthestimulator
(2)andremovethedepletedbatterypack(Figure7).
B. Followingthearrows,placethedepletedbatterypack
into the battery charger cradle (1, 2) for charging
(Figure8).Asolidorangelightonthechargercradle
willilluminate, indicatingthebattery ischarging. If
nolightappearsonthechargingcradleanerroris
indicated. If this occurs, try removing the battery
packfromthechargercradleandreinsertingit.Ifthe
orangelightdoesnotappearcontactBiomet.
C. Once the charger cradle’s orange light turns off
and a solid green light appears, the battery pack
is fully charged. Remove the battery pack (1, 2)
from the battery charger cradle with a gentle lift
on the battery tab (Figure 9) and place the fully
charged battery pack into the Biomet® SpinalPak®
Non-invasive Spine Fusion Stimulator System in
order to commence treatment.
D. Thereshouldalwaysbeonebatterypackinthecharger
andonebatterypackinstalledinthestimulatoratall
times,ensuringafullychargedbatterypackevery24
hoursasrecommended.
NOTE: Do not be concerned if the battery packs are inadvertently charged more than once or kept on
the charger cradle for a long period of time. The battery packs cannot be overcharged. If the battery
pack is in the battery pack charger and the battery pack is fully charged, the charger will terminate
the recharging process. The charger cradle will indicate termination of charging when the orange
light is not illuminated. Additional replacement battery packs are available by contacting Biomet.
Figure 7
Figure 8
Figure 9

8 9
Button Functions
ALARM ON/OFF BUTTON
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System is activated as soon as a
chargedbatterypackisinserted.ThebuttonlocatedbelowtheLCDdisplayenablesordisablesthe
audiblealarm.Duringanalarmcondition,depressingthebuttonquickly(0.5seconds)willtemporarily
disabletheaudiblealarm.Depressingthebuttonforalongerperiodoftime(3seconds)willtoggle
theaudiblealarmbetweenenabledanddisabled.Patientsshouldbeadvisedtoleavetheaudiblealarm
enabledasfrequentlyaspossibleinordertoassurethefullyprescribedtreatment.Aspeakersymbol
willbeindicatedontheLCDdisplaywhenthealarmisenabled.
Thealarmdefaultstoaudiblealarm.Pressthebuttonbelowthedisplayonthefrontofthestimulator
tosilencethealarm.Aftersilenced,thelightwillcontinuetoflashandthedisplaywillindicatethe
alarmcondition.
Symbol Condition Instructions
Treating Continueuse.
Audiblealarm Ifbeeping,depressthebutton
brieflytosilencethealarm.Depressthe
buttonapproximately3secondsto
engageordisengagetheaudiblealarm.
Lowbatterycharge Insertachargedbatterypack.
Disconnection Confirmthateachelectrodeisproperly
appliedontheskin.Seetheelectrode
pouchforinstructions.Confirmthatthe
leadwireisattachedproperly.Replace
theleadwireifnecessary.
Systemerror Errorinthestimulator–
ContactBiometforassistance.
Stimulatoris StimulatorwillnottreatuntilUSB
connectedto cableisdisconnected
a PC
Endofoperation/ ContactBiomet
TreatmentCompletion
LCD Symbol Descriptions and Instructions

10 11
Treatment Completion
Therapeutictreatmentshouldnotbesuspendeduntilfusionoccursoruntilsuchtime
asadeterminationismadebytheprescribingphysician.Thedeviceisprogrammedto
deliver 270 continuous days of therapeutic treatment and automatically discontinues
operationafterthe270days.
Patient Compliance Monitoring
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemcontainsembeddedsoftware
whichallows the displayof patientspecific historydata including usageand therapeutictreatment
times.Thisdatamaybedownloadedtoapersonalcomputerforviewing,storageand/orprintoutvia
theuseofBiometComplianceDataDownloadSoftware.PleasecallyourlocalBiometrepresentative
toobtainmoreinformation.
Ordering Information
Toordersupplies,contactBiomet.Seepage2“ImportantSafeguards”forcontactinformation.
The following information is necessary to expedite any inquiry:
• Patientname
• Physicianname
• Addresstosendreplacementparts(patienthome,MDoffice,etc.)
Electrode Instructions for Use
Donotopenouterpacketuntilreadytouse.
1) Tearopenpacket.
2) Removeelectrodefromclearplasticbackingliner.
3) Wetfingerwithtapwaterandmoistenentiregelarea.
4) Placeelectrodeonskin.
5) Connectelectrodetoelectrodelead.
Renewal
1) Withcontinuoususe,electrodesmaydryout.
2) Torenew,wetfingerwithtapwaterandmoistenentiregelarea.
3) Reapplyelectrodetoskin.
Storeinacoolplace

10 11
Attentionseeinstructions
AlternatingCurrent
DirectCurrent
TypeB
Storage/Transport
temperaturelimits
Class II
NonSterile
Manufacturer
WEEE
SinglePatientUse
PrescriptionOnly
Warning:Theconcomitant
useofthestimulatoranda
pacemakerorcardioverter
mustbeassessedbya
cardiologistonan
individualbasiswithan
Electrocardiogram(EKG).
Caution:Thesafetyof
thisdeviceusedduring
pregnancyandnursing
inhumanshasnot
beenestablished.
Symbol Description
Equipment Classification
• Stimulator-Internallypoweredbyrechargeablebatteries
• Charger-ClassII,TypeB
• OrdinaryEquipmentwithoutprotectionagainstingressofwater
• Equipmentnotsuitableforuseinpresenceofflammableanestheticmixturewithairoroxygen
ornitrousoxide.
• Modeofoperation-continuous
Cleaning Instructions
UseadampclothforcleaninganypartoftheBiomet®SpinalPak®Non-invasiveSpineFusion
StimulatorSystem.Donotusecleaningproductsordetergents.
2
Rx only
12 13
RFemissions
CISPR 11
Electromagnetic Compatibility
A. Theuseofaccessories,cablesorreplacementpartsotherthanthosesuppliedbyBiometmay
resultinincreasedemissionsordecreasedimmunityoftheequipmentorsystem.
B. Thisequipmentshouldnotbeusedadjacenttoorstackeduponotherequipment.
C. PortableandmobileRFcommunicationsequipmentcanadverselyaffecttheoperationof
MedicalElectricalEquipment.
D. Intheeventthisequipmentinterfereswiththeoperationofotherequipment,orexperiences
interferencefromotherequipment,tocontinuetreatment,itwillbenecessarytomovethe
Biomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemawayfromthesourceofthe
interferenceasindicatedinTable4.
Guidance and manufacturer’s declaration -
electromagnetic emissions
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemisintendedforuseinthe
electromagneticenvironmentspecifiedbelow.ThecustomerortheuseroftheBiomet®SpinalPak®
Non-invasiveSpineFusionStimulatorSystemshouldassurethatitisusedinsuchanenvironment.
Emissionstest Compliance Electromagneticenvironment-guidance
Group 1
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulator
SystemusesRFenergyonlyforitsinternalfunction.Therefore,
itsRFemissionsareverylowandnotlikelytocauseany
interferenceinnearbyelectronicequipment.
RFemissions
CISPR 11
Class B
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulator
Systemissuitableforuseinallestablishments,including
domesticestablishmentsandthosedirectlyconnectedtothe
publiclow-voltagepowersupplynetworkthatsupplies
buildingsusedfordomesticpurposes.
Harmonic
emissions
IEC 61000-3-2
Notapplicable
Voltage
fluctuations/
flickeremissions
IEC 61000-3-3
Notapplicable
Table 1

12 13
Guidance and manufacturers declaration - electromagnetic immunity
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemisintendedforuseinthe
electromagneticenvironmentspecifiedbelow.ThecustomerortheuseroftheBiomet®SpinalPak®
Non-invasiveSpineFusionStimulatorSystemshouldassurethatitisusedinsuchanenvironment.
Immunitytest IEC 60601
testlevel
Electromagneticenvironment-
guidance
Electrostatic
discharge(ESD)
IEC 610004-2
± 6 kV contact
±8kVair
Floorsshouldbewood,concreteor
ceramictile.Iffloorsarecoveredwith
syntheticmaterial,therelativehumidity
shouldbeatleast30%.
Electricalfast
transient/burst
IEC 61000-4-4
NotApplicable
Surge
IEC 61000-4-5
Notapplicable
Voltagedips,short
interruptionsand
voltagevariations
onpowersupply
inputlines
IEC 61000-4-11
Notapplicable
Powerfrequency
(50/60Hz)
magneticfield
IEC 61000-4-8
Notapplicable
Compliancelevel
Group 1
Class B
Notapplicable
Notapplicable
Notapplicable
Table 2

14 15
Guidance and manufacturers declaration - electromagnetic immunity
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemisintendedforuseinthe
electromagneticenvironmentspecifiedbelow.ThecustomerortheuseroftheBiomet®SpinalPak®
Non-invasiveSpineFusionStimulatorSystemshouldassurethatitisusedinsuchanenvironment.
Immunitytest IEC 60601
testlevel
Electromagneticenvironment-
guidance
Conducted RF
IEC 61000-4-6 NotApplicable
NOTE1. At80MHzand800MHz,thehigherfrequencyapplies.
NOTE2. Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationisaffectedbyabsorptionand
reflectionfromstructures,objects,andpeople.
aFieldstrengthsfromfixedtransmitters,suchasbasestationsforradio(cellular/cordless)telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters,anelectromagneticsitesurveyshouldbeconsidered.Ifthemeasuredfieldstrengthinthe
locationinwhichtheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemisusedexceeds
the applicable RF compliance level, the Biomet® SpinalPak®Non-invasive Spine Fusion Stimulator
Systemdeviceshouldbeobservedtoverifynormaloperation.Ifabnormalperformanceisobserved,
additional measures may be necessary, such as reorienting or relocating the Biomet® SpinalPak®
Non-invasiveSpineFusionStimulatorSystem.
bOverthefrequencyrange150kHzto80MHz,fieldstrengthsshouldbelessthan1V/m.
Compliancelevel
NotApplicable
RadiatedRF
IEC 61000-4-3
3V/m
80MHzto2.5
GHz
1V/m PortableandmobileRFcommunicationsequipment
shouldbeusednoclosertoanypartoftheBiomet®
SpinalPak®Non-invasiveSpineFusionStimulatorSystem,
includingcables,thantherecommendedseparation
distancecalculatedfromtheequationapplicabletothe
frequencyofthetransmitter.
Recommendedseparationdistance
d=3.5√P80MHzto800MHz
d=7√P800MHzto2.5GHz
wherePisthemaximumpoweroutputratingofthe
transmitterinwatts(W)accordingtothetransmitter
manufactureranddistherecommendedseparation
distanceinmeters(m).
FieldstrengthsfromfixedRFtransmitters,as
determinedbyanelectromagneticsitesurvey,ashouldbe
lessthanthecompliancelevelineachfrequencyrange.b
Interferencemayoccurinthevicinityofequipment
markedwiththefollowingsymbol:
Table 3

14 15
Recommended separation distances between portable and
mobile RF communications equipment and the Biomet®
SpinalPak®Non-invasive Spine Fusion Stimulator System
TheBiomet®SpinalPak® Non-invasiveSpineFusionStimulatorSystemisintendedforuseinan
electromagneticenvironmentinwhichradiatedRFdisturbancesarecontrolled.Thecustomerortheuser
oftheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemcanhelppreventelectromagnetic
interferencebymaintainingaminimumdistancebetweenportableandmobilecommunicationsequipment
(transmitters)andtheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystem as recommended
below,accordingtothemaximumpoweroutputofthecommunicationsequipment.
Rated maximum
output power of
transmitter
W
Separation distance (meters)
according to frequency of transmitter
150kHzto80
MHz
d = 3.5 √ P
80MHzto800
MHz
d = 3.5 √ P
800MHzto2.5
GHz
d = 7 √ P
0.01 .35 .35 .7
0.1 1.1 1.1 2.21
1 3.5 3.5 7
10 11.06 11.06 22.13
100 35 35 70
Fortransmittersratedatamaximumoutputpowernotlistedabove,therecommendedseparation
distancedinmeters(m)canbeestimatedusingtheequationapplicabletothefrequencyofthe
transmitter,wherePisthemaximumoutputpowerratingofthetransmitterinwatts(W)according
tothetransmittermanufacturer.
NOTE1.At80MHzand800MHz,theseparationdistanceforthehigherfrequencyapplies.
NOTE2.Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationis
affectedbyabsorptionandreflectionfromstructures,objects,andpeople.
Table 4

16 17
Patient Counseling Information
ThepatientshouldbethoroughlyinstructedonhowtoproperlyuseandcarefortheBiomet®SpinalPak®
Non-invasiveSpineFusionStimulatorSystemandreceivethePatientManual,whichprovidesdetailed
instructions.Asummaryofthekeypointsinthepatientlabelingisprovidedbelow.
Compliance- Thepatientshouldbeinstructedthatcompliancewithdeviceuseandcareiscritical
toassuretheproperfunctionofthedeviceandeffectivetreatment.
Battery Pack- The patient should be instructed to insert a fully charged battery pack into the
stimulatorevery24hours.
Electrodes- The patient should be instructed to replace the electrodes when needed, and
to clean the electrode sites thoroughly with soap and water prior to applying
theelectrodes.
Skin Irritation - Thepatientshouldbeinstructedtoexaminetheskinforirritationwhenreplacing
theelectrodes.Ifirritationispresent,thepatientshouldbeinstructedtorelocatethe
electrodesadjacenttotheoriginalsites.Thepatientshouldbeevaluatedperiodically
toassesstheskinforsensitivity.
Alarms- See LCD Symbol Descriptions and Instructions (page 9). The patient should be
instructedtokeeptheaudiblealarmsystemengagedasoftenaspractical,andto
engagethealarmsystemifithasbeendisengagedassoonaspractical.
Bathing- The patient should be instructed to disconnect the stimulator during bathing,
showering or swimming. It should be reconnected as soon as practical
followingthese activities.Thepatient shouldalso beinstructedto eitherremove
the electrodes, or to cover the electrodes with the protective retainer patches,
duringshowering.
Storage and Handling
TheBiomet®SpinalPak®Non-invasiveSpineFusionStimulatorSystemshouldbestoredinacooland
dryplace.Thedevicecomponentsshouldbehandledwithcare.Damagemayoccurifthedeviceis
inappropriatelyhandledorabused.
Disposal Instructions
When treatment has concluded as determined by the prescribing physician (see page 10), Biomet
requests that the patient disposes of the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator
Systemaccordingtolocalstatutesandregulations.

16 17
Notes:

OPTIONS
Theindustry’smostcomprehensiveoptions:
• PEMF,CCandDC
• Anatomyspecificcoils
• Wear-timechoice
EVIDENCE
• Backedbyprovenscience
• Multiplescientificpapers
• Theproofisinthepatient
EXPERIENCE
Recognizedasanindustry
pioneerwithEBIlineage,Biomet
hashelpedoveronemillionpeople
Tolearnmoreaboutthisproduct,
contactyourlocalBiometSalesRepresentativetoday.
PN#1067795-00Rev.D
Biomet®SpinalPak®Non-invasive
Spine Fusion Stimulator System
Complete Manual and Package Insert
399JeffersonRoad•Parsippany,NJ07054
800.526.2579•www.biomet.com•BNS231002L11/13
©2013EBI,LLC.AlltrademarksarethepropertyofBiomet,Inc.
oroneofitssubsidiariesunlessotherwiseindicated.RxOnly.
Table of contents
Popular Medical Equipment manuals by other brands

BodyMed
BodyMed ZZA250 instruction manual

Otto Bock
Otto Bock 4R63 Instructions for use

Invivo
Invivo dS HiRes Hand/Wrist 16ch 1.5T Instructions for use

KOKEN
KOKEN LM-107C instruction manual
Ormesa
Ormesa Birillo Use and maintenance handbook
Smiths Medical
Smiths Medical Surgivet Advisor Quick reference guide