Bionicare KNEE SYSTEM User manual

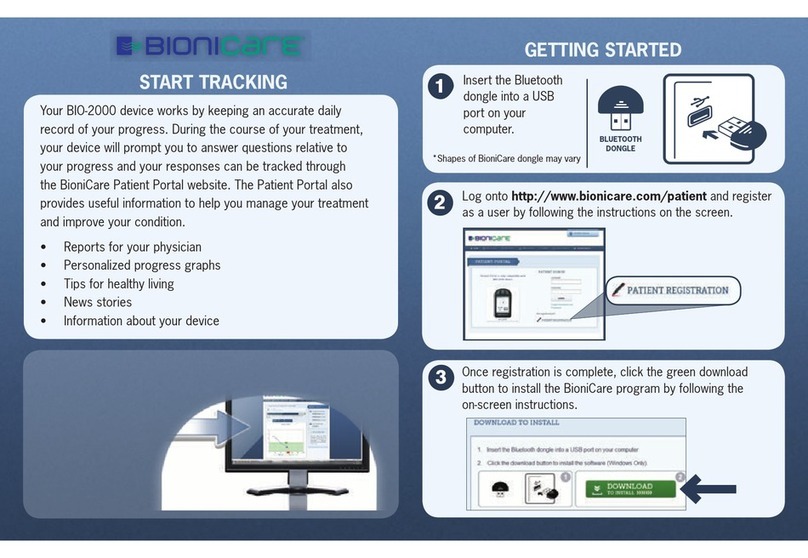
QUICK START GUIDE
BIONICARE KNEE SYSTEM

Congratulations on your BioniCare Knee System—
the only non-invasive treatment for osteoarthritis of the knee
that improves the knee, defers knee replacement and reduces medications!
The BioniCare Knee System involves the use of a therapeutic electrical current that mimics the signal
of a healthy knee to treat osteoarthritis. It takes daily use over several weeks to feel a reduction in
pain and an improvement in functioning. The device should be worn subsensory (you should not be
able to feel it). Most patients will
NOT FEEL ANYTHING
even at the maximum intensity. You can
check the device to make sure it is sending the signal out, but you will know that it is working by the
improvements you see in your knee.
If you have any questions about the use of the BioniCare Knee System,
please call our Patient Care Department at 800.452.7993
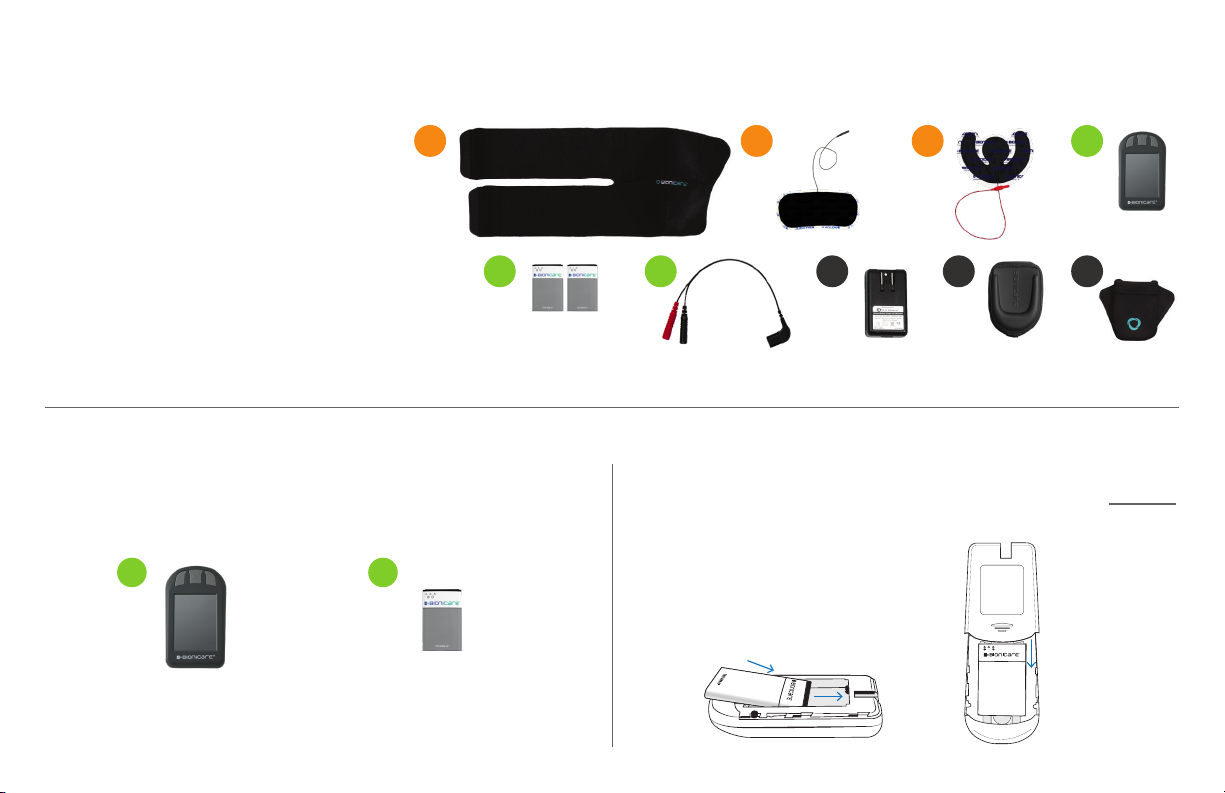
1234
56 7 8 9
45
1 BioniCare
Device
1 Rechargeable
Battery THIS SIDE UP
STEP 1: TAKE THESE ITEMS TO USE IN WRAP. STEP 1A: Press battery door up to open and lift o. INSERT
battery into the device. Slide door to close.
NOTE: These instructions are for using BioniCare in the wrap. Follow the instructions for BioniCare in the OActive 2, OActive Align or
Catalyst•Propel OA for use in your brace.
1. BioniCare Wrap
2. Thigh Electrode
3. Knee Electrode
4. BioniCare Device
5. (2) Rechargeable Batteries
6. Short Lead Wire (Short lead wire for use only
in wrap. Optional extended/long lead wire for
use in brace.)
7. Battery Charger
8. Device Holster
9. Roaming Pocket
YOUR BIONICARE KNEE SYSTEM INCLUDES:
BACK VIEW
SIDE VIEW

REPEAT: Repeat process for thigh electrode so that wire
extends up out of the wrap, (not down towards the knee).
TIP: KEEP plastic sheets to reapply on electrodes after each use.
2
3
1 BioniCare Wrap
1 Knee Electrode 1 Roaming Pocket
1
9
1 Thigh Electrode
6
1 Short Lead Wire
STEP 2: TAKE THESE ITEMS STEP 3: Lay Wrap on table with 1 KNEE and 1 THIGH
electrode.
STEP 4B: Feed knee electrode wire through two guides
labeled 1 and 2, either orange or green depending on which
side wire comes out. Wire should end up coming out through
the top of the wrap, next to the thigh electrode wire.
STEP 4: Peel WHITE backing o knee electrode and apply
within outlines on BioniCare Wrap. Line up electrode with
either orange or green outline so that wire will point towards
inside of your knee when applied.
NOTE: The white side of the electrode should be against the wrap and the
black should be against the skin.
WHITE
SIDE
KNEE
ELECTRODE
THIGH ELECTRODE
GOES HERE
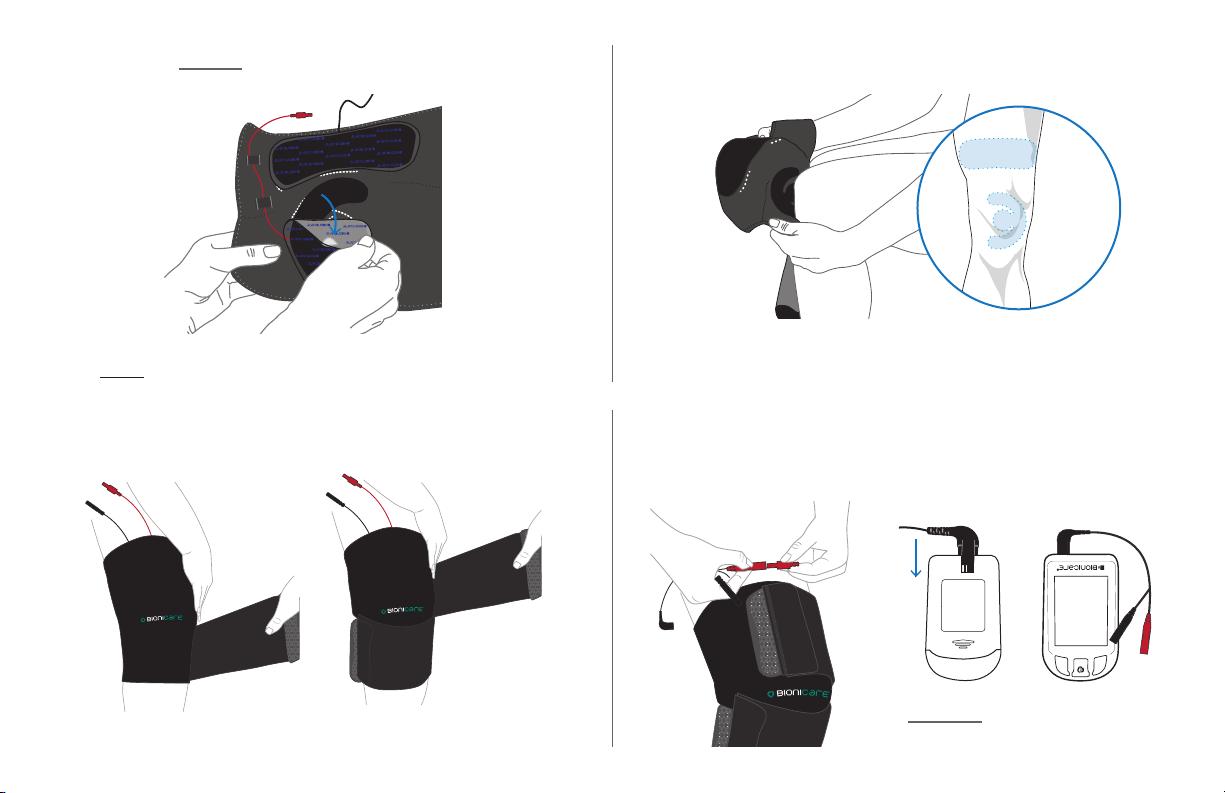
STEP 7: Wrap top and bottom straps around leg.
STEP 6: Apply BioniCare Wrap to knee.
STEP 8: Attach red lead wire tip to the knee electrode and
the black lead wire tip to the thigh electrode. Match red to
red and black to black.
Line up circle of knee electrode over your kneecap and thigh
electrode over your thigh with electrodes sticking to skin.
Wires should extend upward from the BioniCare Wrap.
FRONT BACK
LEAD WIRE PORT
INSERT lead wire into the
BioniCare device.
TIP: KEEP plastic sheets to reapply on electrodes after each use.
STEP 5: Peel CLEAR backing o each electrode.
KNEE
KNEE
THIGH
CALF
SIDE
VIEW

STEP 11: Press play button to start treatment.STEP 10: Answer the following questions.
Your device will periodically ask 4 questions to chart your
progress. Press the middle button to answer and the side
buttons to scroll through answer options.
TIP: You can press the green play button
or middle button to start treatment.
STEP 9: Press middle button to turn on device. Press the
unlock icon on screen to unlock.
TIP: Middle button will also unlock screen.
STEP 8A: Attach the roaming pocket on the front of the
brace in a comfortable location.
Recommended treatment time is a minimum of 8 hours a day. However, the more hours you treat with the BioniCare System, the sooner you
will see improvements in your knee.
NOTE: If your device says “OPEN” after you get past 2.0 volts, the device is not connected correctly or not making good contact.
• Make sure white side of electrode is against wrap and black gel is against skin (STEPS 4-5).
• Make sure electrodes are stuck to skin (STEP 6) and wires are all connected (STEP 8).
• If electrodes are old, replace your electrodes.
If you cannot get the OPEN error to stop, try the troubleshooting section of the manual or call Patient Care at 800.452.7993.
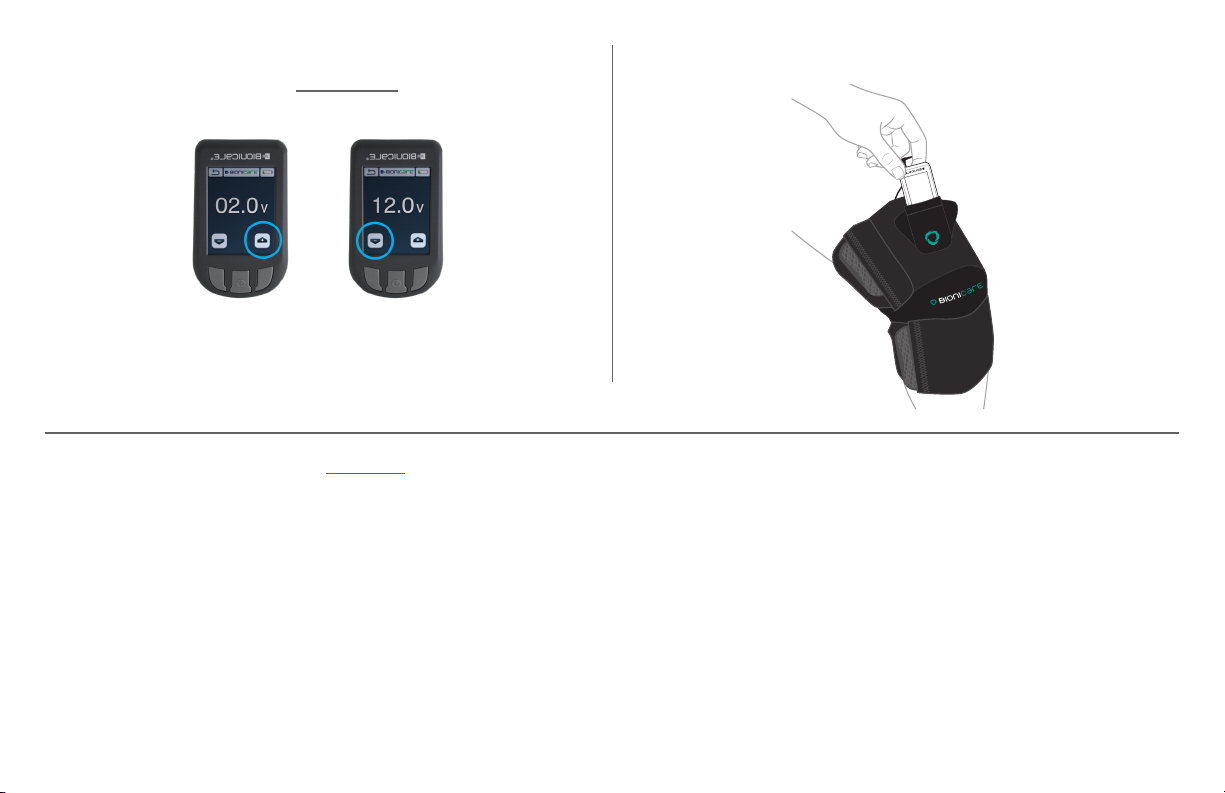
STEP 12A: Place device in roaming pocket. Tuck wires as
needed.
STEP 12: Increase past 2.0 volts then continue to increase
until you feel tingling. Then DECREASE until you no longer
feel tingling.
It’s okay if you do not feel tingling even at 12.0 volts.
The BioniCare device is still administering treatment.
To save battery life, set treatment at 8.0 volts.

ELECTRODES
• Always replace clear plastic backings on electrodes after each use.
• Do not use alcohol to clean skin surface.
• Electrodes should only be used on dry, clean, unbroken skin surfaces. Prior to
BioniCare Wrap application wash skin with soap and water and completely dry.
• Do not apply skin lotion prior to application of BioniCare Wrap.
• Do not trim electrodes, as cut edges may aect the even distribution of stimulation.
• Remove BioniCare Wrap before showering.
• If you experience symptoms of skin irritation while using the BioniCare system,
discontinue use immediately and contact VQ OrthoCare’s Patient Care Department
at 800.452.7993, or consult your BioniCare provider or healthcare practitioner.
CAUTION
Electrodes should be discarded and replaced if damaged or if they show signs
of wear. If in doubt about the integrity or proper function of any electrode,
replace it before proceeding with treatment.
In addition, refer the patient to the manual to read the BioniCare Generator
Cautions and Care and Cleaning.
CARE AND MAINTENANCE
CHARGING THE BATTERY FOR THE BIONICARE GENERATOR:
The BioniCare Generator is powered by a single rechargeable Lithium Ion
battery. The battery charger can be used at 110 or 220 volts and fully charges
the battery in four hours. Use only the charger supplied by VQ OrthoCare as
improper charging can cause heat damage or even high pressure rupture.
Do not use any battery that shows any signs of corrosion, leaking or other
damage. Replace corroded, leaking or damaged batteries.
LOW BATTERY:
When the device determines the battery is approaching the end of its usable
charge, the device will continue to operate but the battery icon on the main
display screen will flash and audible beep will sound. When this occurs, recharge
the battery or replace it with a freshly charged battery.
BIONICARE WRAP
To clean BioniCare Wrap, the electrodes should first be removed. The BioniCare
Wrap should be hand washed in cold water with mild detergent. Rinse thoroughly
and air dry (do not machine dry). Never machine wash the BioniCare Wrap. Never
use bleach, fabric softener, or harsh detergents.
FOR QUESTIONS OR TROUBLESHOOTING PLEASE CALL 800.452.7993 TO SPEAK TO A BIONICARE REPRESENTATIVE.
INDICATIONS: The BioniCare Knee System is indicated for
use as an adjunctive therapy in reducing the level of pain and
symptoms associated with osteoarthritis of the knee and for
overall improvement of the knee as assessed by the Physician’s
Global Evaluation (clinical studies).
CONTRAINDICATIONS
• Do not use the BioniCare Knee System for any electrode place-
ment that applies current to the carotid sinus (neck) region.
• Do not use the BioniCare Knee System for any electrode place-
ment that causes current to flow transcerebrally (through the
head).
• Do not use the BioniCare Knee System when pain syndromes
are undiagnosed until etiology is established.
PRECAUTIONS: Isolated cases of skin irritation may occur at
the site of electrode placement following long-term application.
ADVERSE REACTIONS: Skin irritation and electrode burns
are potential adverse reactions. Patients with skin irritation/
reactions should be monitored.
WARNINGS
• The BioniCare Knee System must be used only as prescribed
and applied only to the knee.
• Patients with demand style cardiac pacemakers should
consult with their physician prior to use of this system.
• The safety of the BioniCare Knee System for use during
pregnancy has not been established.
• The BioniCare Knee System is not eective for pain of central
origin (including headache).
• Use only under the continued supervision of a physician.
• Keep out of reach of children.
• Electronic monitoring equipment (such as ECG monitors and
ECG alarms) may not operate properly when the BioniCare
Knee Device is in use.
If you experience pain, swelling, sensation changes, or unusual
reactions while using this product, contact VQ OrthoCare’s
Patient Care department at 800.452.7993 or consult a
physician.
CAUTION: Federal law restricts this device to sale by or on the
order of a practitioner licensed by the law of the State in which
he/she practices to use or order the use of the device.

NOTES:

NOTES:

NOTES:

© 2022 VisionQuest Industries, Inc. | VQO511971 REV F
1390 Decision St., Suite A • Vista, CA 92081 • USA
800.266.6969 • www.vqorthocare.com
NOTES: BIONICARE KNEE SYSTEM
QUICK START GUIDE
VERSION EN ESPAÑOL
BIONICARE KNEE SYSTEM
USER MANUAL
ENGLISH/ESPAÑOL
Other manuals for KNEE SYSTEM
1
Table of contents
Other Bionicare Medical Equipment manuals