Boehringer 3708 User manual

3708.003 Rev G P/N 33397 1 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
User Manual
Boehringer Laboratories, LLC
300 Thoms Dr.
Phoenixville, PA 19460
800-642-4945
MRI Suction
Regulator Model:
3708
MRI SUCTION REGULATOR
3800
3804
3840

3708.003 Rev G P/N 33397 2 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
Congratulations on your purchase of a Boehringer Suction Regulator. We consider
our Suction Regulators to be the best in the world. We are confident it will provide
you with reliable, trouble-free, safe patient care and low cost of operation. This
product is intended for use by individuals properly trained in suctioning procedures by
or on the order of a physician. Please read these instructions carefully.
Table of Contents
Clinical Use……………….…………………………………………………………………... 4
Warnings…………….…………………………….…………………………………………… 4
Caution…………………….…………………………….…………………………………….. 4
Installation ………………………….…………………………….…………………………… 5
Operation ……………………………………………………………………………………. 5
Maintenance……..………………………………….…………………….…………………… 5
Cleaning and Disinfection………………………………….…………………….…………… 6
Disassembly …………………….……………….…………………………….…………..…. 6
Assembly and Lubrication……………………………….……………………………………. 7
Test ……………………………………………….……………………………………………. 8
Troubleshooting….……………………………………….……………………………………. 9
Specifications…….……………………………………………………….……………………. 9
Operating and Storage Limits….………………………………….…………………………. 9
Warranty and Repair……………………………………………………….…………………. 10
Exploded Views with Replacement Parts List………………………………………………. 11
Suction Fittings ………….……………………………………………………………………. 12
Accessories………..….………………………………………………………….……………. 12

3708.003 Rev G P/N 33397 3 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
VACUUM Air or other gases at a sub atmospheric pressure typically expressed as mmHg or cmH20.
SUCTION A use of vacuum that causes a fluid or solid to be drawn into an interior space or to adhere to a
surface because of the difference between the external and internal pressures.
Alerts the user to the presence important operating and maintenance instructions in the
literature accompanying the device.
WARNING Alerts user to actions or conditions that could result in injury to user or patient.
CAUTION Alerts user to actions or conditions that can cause damage to the device or may result in
substandard performance of the device or system.
IMPORTANT Indicates an action that is emphasized to ensure proper operation of equipment.
OFF Supply suction is off and patient circuit is vented to atmospheric pressure.
REG Supply suction is on and regulated output is controlled to prescribed setting.
LINE Supply suction is on and regulation is bypassed to deliver maximum suction to collection circuit.
CONTIN Intermitting regulator setting that provides continuous regulated suction to the collection circuit.
INT Intermitting regulator setting that allows periodic, automatic application & venting of the
collection circuit.
Lo Spike: Accuracy of regulation depends primarily on the ability to provide a consistent level
of vacuum under changing flow conditions.
Involuntary pneumatic biopsy, or tissue damage, can occur when high levels of vacuum are
applied to delicate tissue. With a Boehringer regulator, you can depend on very low “spike”
compared to our competitor’s models.
"Spike" is the variation in indicated suction as flow in the collection circuit changes from an a
free flowing condition to an occluded condition. We measure spike as the change in indicated
suction from full flow to a no flow condition using a typical collection circuit with a 14 French
catheter. To test, set the regulator to 100 mm flowing, and then allow occlude the 14Fr
catheter. The change in the indicated suction level is "Spike".
Boehringer regulators are checked on the assembly line to meet a specification of less than
10% of the indicated setting, for example 10mmHg spike at a 100 mmHg setting.
An evaluation of a regulator’s spike allows one to determine whether the device is truly
“regulating”. A safe and reliable regulator should regulate to its set position regardless of
variable flow conditions.
PARALLAX Inaccuracy caused by observational position of an indicating element (pointer) to a reference
element (scale).
Definition of Terms and Symbology

3708.003 Rev G P/N 33397 4 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
▪This product is intended for use by or on the order of a physician. It is to be used by individuals who are
properly trained in medical suctioning procedures. Please read these instructions carefully.
▪Suction regulators must only be attached to vacuum systems. Do not attach to compressed air,
nitrogen, or oxygen sources.
▪Suction catheters, collection canisters and suction tubing must be carefully evaluated and
selected to ensure adequate function for the specific clinical environment and intended field of
use.
▪Do not use Boehringer suction regulators in the presence of flammable anesthetics.
▪Do not use conductive tubing in an MRI environment. To avoid inductive heating, do not loop collection
tubing.
▪There have been reports of increased intracranial pressures associated with endotracheal suctioning
procedures. "Persistent, increased ICP has been associated with neurological damage and fatalities".
(ECRI, Healthcare Product Comparison System (US). Regulators, Suction. Plymouth Meeting (PA),
1999.).
Always verify regulator operation (Droop, see page 4 for details) before use on a patient. Verify
operation by establishing the desired vacuum level with the collection circuit and suction
catheter attached to the regulator. Occlude the suction catheter and note that the indicated
vacuum does not rise by more than 10% of the original setting.
▪Intermitting Regulators: The automatic return of the patient circuit to atmosphere may not
eliminate the need for catheter tip irrigation. As with any automatic system, it is important to
monitor the results to be sure that drainage is occurring in a safe efficient manner. The fact that
the intermitter is cycling is not an indication that effective drainage is occurring.
▪The Model 3708 Boehringer MRI Suction Regulator is the only MR conditional regulator with FDA
510K approval.
▪The high air flow of the models 3740 and 3840 contraindicate their use with a Vacuum Assisted
Delivery (VAD).
▪Collection canisters are mandatory for all suction procedures. We strongly recommend the use of an
overflow protection device (filter or trap bottle) at all times between the regulator and collection canister.
See Accessories at the end of this manual.
The 3700 Series Continuous Suction Regulators are designed to provide accurate control of wall suction for
use in suction therapy procedures in the operating, recovery, intensive care unit, labor and delivery, neonatal,
pediatrics, patient bedside, and emergency room.
Safety Information
WARNING!
CAUTION!
CLINICAL USE

3708.003 Rev G P/N 33397 5 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
The 3700 Series Intermitting Suction Regulators are designed to provide an intermittent suction source for
gastrointestinal drainage procedures. They may also be used in procedures needing continuous suction.
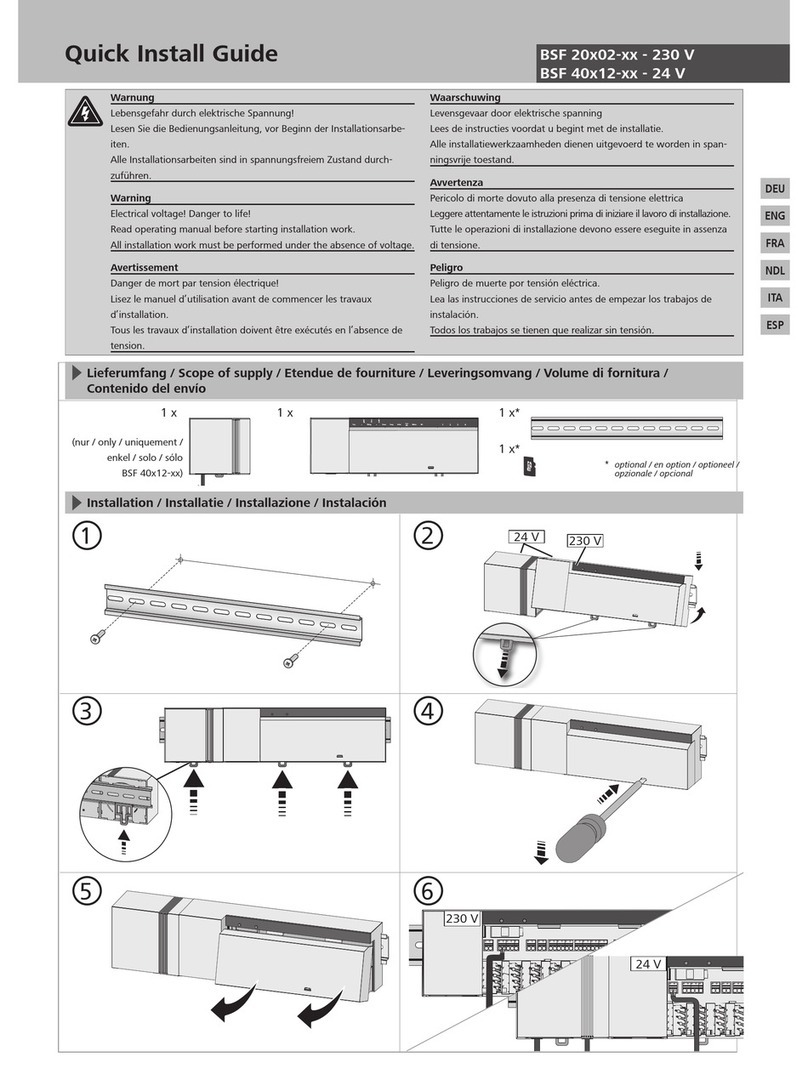
Installation
All Boehringer MRI Conditional suction regulators are supplied with fittings specified at the
time of purchase. These fittings are not to be removed or replaced by anyone other than
Boehringer Laboratories personnel. Removal or replacement of any MRI Conditional suction
regulator fitting by other than Boehringer Laboratory personnel could create a safety hazard,
and will void any and all warranties.
Operation –
Model 3708 MRI Regulator incorporates a 3-way control valve with OFF, REG, and LINE.
OFF: With control valve in the OFF position, suction is off and the collection circuit is returned
to atmospheric pressure by an internal vent port, a special feature of the Boehringer design.
REG: With control valve in the REG position, wall suction may be controlled to a specific
level by turning the large adjusting knob in the direction indicated. A spring opposed
diaphragm assembly precisely controls the level of suction provided at the lower port of the
Regulator within the range of the gauge. This assembly "senses" changes in the patient
collection circuit and makes appropriate adjustments to maintain the suction level that has
been selected. Regulated settings are verified by the large, easy to read gauge.
LINE: With control valve in the LINE position, the regulating mechanism is bypassed. Full
wall suction is applied to the patient collection system through the lower port of the Regulator.
The LINE suction mode is engaged by depressing the safety spring release and rotating the
control valve to the line position. LINE setting is verified by the exposure of the "LINE"
warning at the bottom of the gauge label.
CAUTION: Full line suction may cause damage to sensitive tissue.
Maintenance - All Models
Your Boehringer Regulators have been designed to provide years of trouble free operation.
Most service activity is the result of aspiration of bodily fluids or other foreign materials into
the regulator. The routine use of an appropriate collection canister greatly reduces needed
service. To determine your cleaning/maintenance schedule:
•Periodically inspect the overall condition of the instrument. Test the gauge accuracy
and check the instrument function as described under ‘Test’. Simply clearing the small
orifices in the gauge view tube and the regulator body can remedy many performance
conditions (see troubleshooting). Return to service if the instrument performs
appropriately per the ‘Test’ requirements.
•Based on data from your periodic inspections, determine a cleaning/maintenance
schedule appropriate for the operational conditions of your facility. Clean, inspect,
lubricate, and test based on your schedule and according to the Instrument Cleaning
and Disinfection, Instrument Lubrication and ‘Test’ section outlined below.

3708.003 Rev G P/N 33397 6 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
Please refer to Boehringer document 7700.192, Boehringer Suction Regulator Recommendations for
Decontamination and Autoclaving for guidance. This information is available online at
www.boehringerlabs.com, or toll-free at 1-800-642-4945.
Regulator:
1. Back out lock screw (8) on housing assembly (7) with 1/16 hex wrench.
2. Unscrew diaphragm housing (7) from regulator body (12).
3. Remove valve retaining screw (9) and washer (10) with 5/32 hex wrench. Pull out
control valve (15).
4. In the unlikely event suctioned material should enter the regulator diaphragm housing,
it will be necessary to disassemble and scrub the unit as follows:
a. Remove quad ring (5), lens cap retaining ring (1) and lens cap (2).
b. Push out diaphragm (3) by pressing on piston/stem assembly (4).
c. Remove piston/stem assembly (4) and spring (6).
Gauge:
5. Remove the retaining ring (17) using retaining ring pliers.
6. From the front of the gauge, use thumb to rotate and loosen view tube (19). Then
remove view tube-piston-diaphragm assembly (19-20-21).
7. Remove the lower lip of the diaphragm from outside of the view tube, and slide out the
diaphragm-piston assembly.
8. Remove the upper diaphragm lip from the top of piston, and slide diaphragm off piston.
9. Remove spring (22).
NOTE: It is not necessary to remove the gauge body (24) from the regulator body (12)
prior to cleaning.
IMPORTANT:Always clean the unit prior to assembly. See the Cleaning and
Disinfection section above for details.
Assembly and Lubrication –
After disassembling and cleaning the instrument, assemble and lubricate as follows. Parts
are available from Boehringer Labs and may be ordered by part number (P/N). Part numbers
are found in figure 1 at the end of this manual.
1. Lubricate control valve (15) over entire mating surface with synthetic lubricant (P/N
1895).
2. Rotate valve as you insert it into the body. Remove the valve and inspect for dry
areas. The valve must have a thin layer of lubricant over its entire diameter without
excess in the cross ports.
3. Lubricate the threaded sleeve in diaphragm housing (7) and U-cup (11) with a light
coat of synthetic lubricant (P/N 1895).
4. Assemble diaphragm housing in reverse order of disassembly.
DISASSEMBLY
Cleaning & Disinfection

3708.003 Rev G P/N 33397 7 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
5. Inspect quad ring seal (5) for cuts or wear. Replace if needed.
6. Assemble unit in reverse order of disassembly.
Gauge:
7. Slide large end of diaphragm (20) over piston (21) until top ring snaps into groove in
piston. Be certain the top bead of the diaphragm is completely engaged into the
groove of the piston.
CAUTION:If the diaphragm is not seated properly it will rub against the view tube and
may lead to premature failure of the gauge.
8. Slide view tube over diaphragm-piston assembly.
9. Roll edge of diaphragm around bottom of view tube and into groove. Make sure there
are no folds or twists and that diaphragm is smooth. Ref. 3700.051 for detailed
assembly instructions.
10.Insert spring (22) into piston/view tube subassembly.
11.Slide piston subassembly with spring up (gauge body facing down) into gauge body
(24).
12.Press until view tube assembly rests on shoulder in gauge body.
13.Assemble retaining ring (17) into groove in gauge body.
Regulator:
14.Place spring (6) on piston/stem assembly (4) and then insert through diaphragm
housing assembly (7).
15.Cover piston/stem assembly (4) with lens cover (2) and snap lens cap retaining ring
(1) into groove.
16.Assemble quad ring (5) to stem (4), then screw housing into body (12).
17.Tighten lock screw (8) to retain diaphragm housing on regulator body groove.
18.Insert the control valve (15), and position the valve retaining washer (10) before
securing it with the valve retaining screw (9)
IMPORTANT:Always test the reassembled unit after each maintenance procedure. See
the Test section below for exact test procedure.
Test –
1. With the control valve in the REG or CONTIN position and a collection system
attached with a 14 Fr. catheter, regulator should control vacuum from 10-100% of full
scale.
2. With the control valve in the REG or CONTIN position and housing turned all the way
off, with suction port occluded, gauge should read zero.
3. With the control valve in the REG or CONTIN, adjust regulator to the middle of the
scale and occlude the catheter. Gauge movement should be less than 5% of the full
scale of the regulator. This measurement is called droop.
4. The gauge should be accurate to 5% of FS for any measurement within the range of
the scale. If this is not the case, please return the gauge to the manufacturer for
repair/replacement.

3708.003 Rev G P/N 33397 8 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
CAUTION: Inaccurate gauge calibration may lead to a high suction condition applied to
the patient.
5. With the control valve in the OFF position, suction should be at atmospheric and
gauge should read zero. With suction port occluded, gauge should read zero.
6. With the control valve in the REG or CONTIN position, set the Regulator to the middle
of the scale and turn control valve to OFF, then back to REG or CONTIN. Gauge
indicator should not travel more than 20% past the set point before settling at the
desired level.
7. A final, important step in instrument maintenance is the identification of the instrument.
This confirms that a qualified individual performed service to accepted procedures and
approved master gauges. An ID tag should accompany the instrument, which
indicates (as a minimum): date of service, individual performing the service and the
date of next service.
CAUTION:Have the regulator factory serviced if not performing to specifications. See
Warranty and Repair on p.12 for details on getting your instrument factory serviced.
MRI Safety Testing Summary
The Boehringer MRI Regulators were evaluated for compatibility in an MRI environment. The
static magnetic field strength was 3.0 Tesla, passively shielded.
The regulator was placed in the bore of the magnet, and no effect on the operation of the
regulator was detected.
A full test report is contained available by request (Boehringer Document Number 3708.019).

3708.003 Rev G P/N 33397 9 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
Troubleshooting- All Models
Boehringer Suction Regulators have been designed for years of trouble-free service. Should
you experience difficulty that is not the result of damage to the instrument, the most likely
cause is aspiration of dirt and/or fluids into the Regulator.
Complete Technical Manuals and Service Bulletins can be found at
http://www.boehringerlabs.com/support/suction-regulator-technical-manuals.php
Video Tips and Techniques for use and repair can be found at:
http://www.boehringerlabs.com/support/video/product-videos.php
http://www.youtube.com/boehringerlabsllc
Symptom
Probable Cause
Solution
Instrument fails to provide suction at
the patient port.
The supply or patient fittings
are clogged
Replace or clean the fittings
Gauge doesn't respond to changes
in suction (via control valve or
adjustment knob)
Gauge diaphragm is improperly
sealed on the gauge piston
and/or view tube
Disassemble gauge and check the
position of the gauge diaphragm.
Gauge piston is discolored
Material has entered the inside
of the gauge
Instrument is contaminated.
Disassemble and clean the instrument.
Gauge responds slowly to changes
in suction
The small hole in the top of the
View Tube may be plugged.
Using a small diameter probe, pierce
the hole to clear the obstruction.
Instrument will not shut off or exhibits
high droop
Dried fluids may have cut the
quad ring seal.
Replace the quad ring (5) and Test the
unit as per above procedure.
Instrument fails to regulate suction
Piston/Stem surface is binding
with foreign matter
Disassemble and clean the instrument.
Erratic gauge movement resulting
from regulator adjustment
Gauge is not sealed
Make sure retaining ring (17) is seated
in groove on the gauge body and there
is no end play in the view tube (19).
IMPORTANT:Always test the reassembled unit after each maintenance procedure.
See the Test section above for exact test procedure.

3708.003 Rev G P/N 33397 10 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
Boehringer Laboratories, LLC guarantees all 3700 Series LONG LIFE Suction Regulators for
TEN years. Boehringer Laboratories, LLC will also warrant all fittings purchased from and
installed by Boehringer Laboratories, LLC for the same warranty period as the suction regulator
on which they were originally installed.
Boehringer Laboratories, LLC warrants to the original purchaser, new suction regulators purchased directly from
Boehringer Laboratories, LLC or from an authorized dealer or representative. This warranty guarantees the
suction regulators to be free from functional defects in materials and workmanship. We also guarantee that our
suction regulators will meet our published specifications.
All regulators returned for repair shall be clean and free from contamination prior to shipment to Boehringer
Laboratories. This requirement is for the safety of our employees as well as to comply with Federal Law
prohibiting the shipment of unmarked biohazard materials. If units are returned contaminated, a cleaning charge
may result.
A service charge may be assessed on any unit returned that shows evidence of gross abuse.
Boehringer Laboratories, LLC is the only authorized warranty service center for our suction regulators. Any
repair service requesting a return authorization for repair will be asked to provide the name and location of the
original equipment purchaser. If this information cannot be provided, the repair is not covered under warranty
and will be a chargeable repair.
This warranty excludes acts of God, fire, flood and acts of war, terror or insurrection.
This warranty is not transferable from the original purchaser.
Boehringer Laboratories’ sole and exclusive remedy under this warranty is limited to repairing and/or replacing
the suction regulator. There are no other express or implied warranties beyond these warranties set forth above.
At Boehringer Laboratories, we are committed to lowering your suction regulator costs of operation!
▼▼▼▼▼▼▼▼▼
All repairs will be shipped back within five days of receipt of purchase order authorization.
For quality factory service, call 800-642-4945 or 610-278-0900 for your return authorization. Ship returns to:
Boehringer Laboratories, LLC
Repair Department
300 Thoms Dr.
Phoenixville, PA 19460
New Products
We are continually striving to reach higher and higher standards of quality. We value your comments and input
on our suction regulators. If you are pleased with this instrument, please find out more about Boehringer
Laboratories' complete line of suction controls.
Warranty and Repair

3708.003 Rev G P/N 33397 11 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
FIGURE 1 –Models 3708, 3718, 3748
No.
P/N
Description
No.
P/N
Description
1
33408
Lens Cap Retaining Ring, MRI
12
1458HC
Reg Body1, Models 3708
2
33916
Lens Cap Disc, Model 3708
13
1462
Thumb Spring
2
33909
Lens Cap Backing, Model 3708
14
1480
Thumb Spring Retaining Screw
3
1466
Regulating Diaphragm
15
1453HC
Control Valve1
4
33411
Piston/Stem Assembly, MRI
16
353
Control Valve O-Ring
5
1461
Quad Ring
17
33409
Gauge Retaining Ring, MRI
6
33403
Spring, 200 mm Hg, MRI
18
Not Used
7
1871
Diaphragm Housing Ass’ly
19
33040
Gauge View Tube2
8
1479
Lock Screw
20
33042
Gauge Diaphragm
9
1468
Control Valve Retaining Screw
21
33036HS
Gauge Piston
10
1454
Control Valve Retaining Washer
22
33406
Gauge Spring, 200 mm Hg, MRI
11
1484
U-cup
23
33043
Fastener
24
33035AN
Gauge Body with Label, 200 mm Hg
1. The Reg Body and Control Valve are factory matched. Please call for details on replacement.
2. For lot numbers before 01G, only the View Tube is needed to replace an earlier View Tube and View Tube
Cover.

3708.003 Rev G P/N 33397 12 of 13
Released September 2022 User Manual Model 3708 MRI Regulator
Specifications –All Models
•Inlet and outlet fittings: 1/8 NPT
•Gauge accuracy ±5% FSO
•Regulation Accuracy: ±10% F.S. from full flow to zero flow with 14 FR catheter
attached.
•Leak rate in OFF position: less than 1 cc/min
•Free Air Flow: Greater than 38 LPM with regulator set to 100 mmHg at standard
JCAHO supply (305mmHg @ 180 SCFH) and a typical collection circuit with a 14 Fr.
catheter.
•Materials: polycarbonate, hard-anodized aluminum, stainless steel, Buna rubber,
silicone, acetal copolymer, Phosphor Bronze.
Model
Regulation Range
User Selectable Modes
Wt. (lb)*
H x W X D (in)
3708
20-200 mm Hg
Off, Regulated Control, Line (source vacuum)
1.35
5¾ x 2½ x 4
*Regulator weights are without fittings.
Operating and Storage Limits –All Models
We recommend that Boehringer Suction regulators be operated and stored at controlled
conditions that typically reflect the medical facility environment.
ACCESSORIES
DISASSEMBLY
Integral Trap Bottle –Model 9100
This device will protect your regulators and suction system from fluid overflow. The bottle
provides a 60 ml backup overflow volume with shut-off capability provided by a precision
float mechanism.

SETTING THE STANDARD
FOR RELIABILITY
Boehringer Laboratories, LLC •300 Thoms Dr. •Phoenixville, PA 19460
800-642-4945 or 610-278-0900
fax: 610-278-0907
www.boehringerlabs.com or www.autovac.com
email: [email protected]
Copyright 2001 Boehringer Laboratories, Inc.
This manual suits for next models
2
Table of contents
Other Boehringer Controllers manuals
Popular Controllers manuals by other brands

NOVAK
NOVAK GOAT PROFILE - SELECTION & GEARING GUIDE manual

DMC
DMC TSC-30/IC Product specification

ComfortClick
ComfortClick Jigsaw Pro CC-JP-3 manual

Emerson
Emerson Asco Advantage Series Installation, operation and maintenance manual

Shimaden
Shimaden PAC46 Series instruction manual

Allen-Bradley
Allen-Bradley AADvance manual