Bosch+Sohn TM Series User manual

0124
boso TM series
24-hour blood pressure monitor
Instructions for use
Instructions for use
EN

2 3
Device overview ...............................................................................4
Scope of supply with complete accessories........................................4
Icon explanation...............................................................................5
Symbols on the measurement device ................................................6
OLED display....................................................................................7
Introduction .....................................................................................8
Intended purpose .............................................................................9
Side-effects of the blood pressure measurement over 24 hours........10
Scope of application.......................................................................10
Notes/safety instructions ................................................................11
Safety information..........................................................................11
Commissioning ..............................................................................14
Selection and connection of the cuff ...............................................14
Attachment of the protective covers (optional)................................15
Attaching the cuff ..........................................................................16
Performing measurements with boso TM series devices ...................17
Ending the measurement and transferring the measurement data...... 19
Replacing the batteries...................................................................20
Charging the batteries....................................................................22
Longer storage of the device...........................................................23
Error codes.....................................................................................24
After use........................................................................................26
Customer information on the recycling
of commercial waste electrical equipment.......................................27
Obligation to report incidents .........................................................28
Warranty conditions / customer service ...........................................29
Accessories ....................................................................................30
Technical specifications...................................................................31
Test instruction for metrological checks...........................................33
EMC information............................................................................34
Contents

4 5
Scope of supply with complete accessories*
• 24-hr blood pressure monitor
• Transport case
• battery charger
• 2x battery sets with two batteries each
(one set already inserted in the unit)
• Cuffs for adults
- Size M CA91 washable
- Size L CA92 washable
• Waist bag
with removeable carrying belt
and strap
*Scope of delivery varies depending on the version of the unit.
• Instruction manual for:
- boso TM series
- XD profile manager
• Important notes
• Medical Devices Book
• CD-ROM
- boso XD profile manager
• USB connection cable
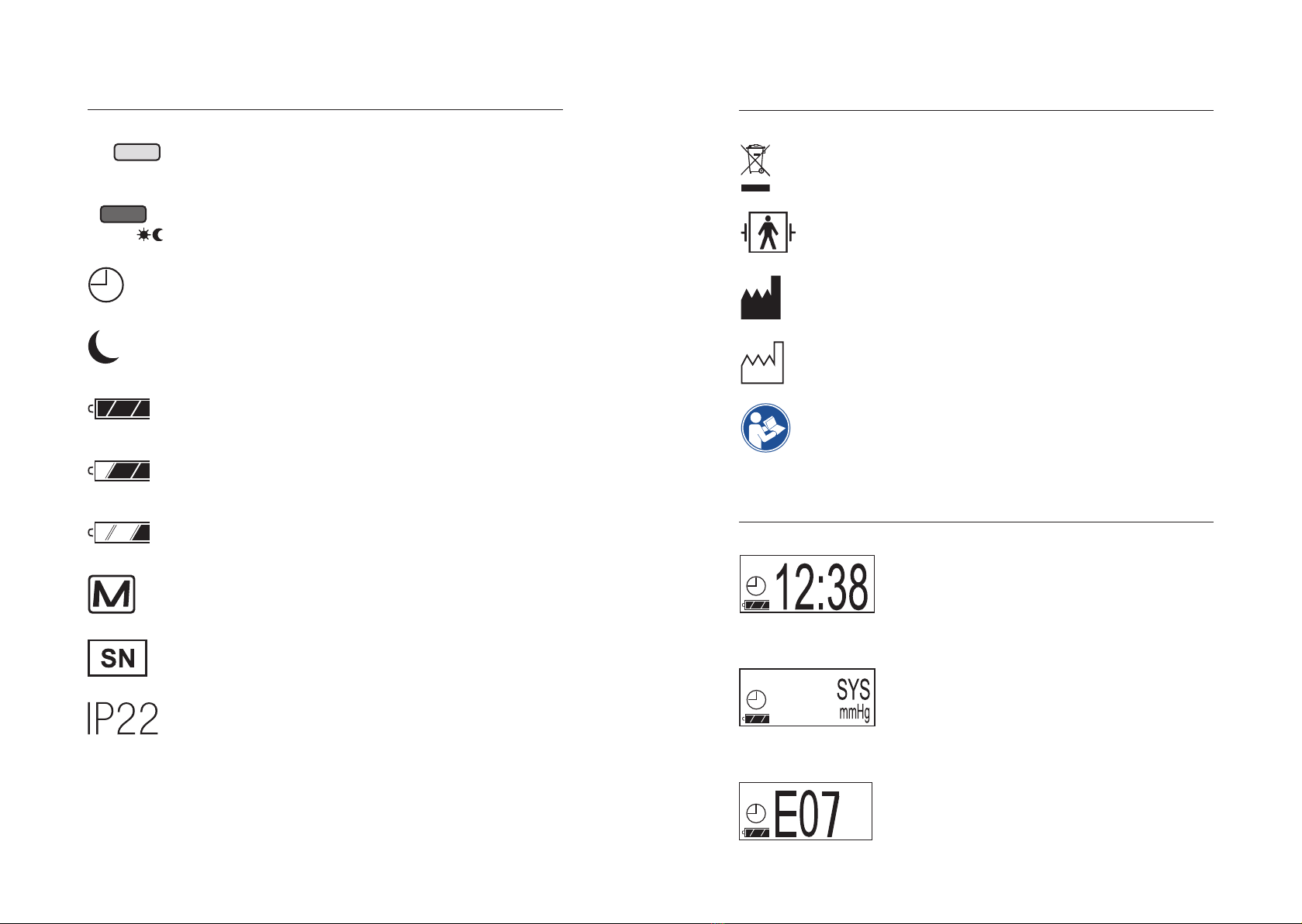
Icon explanation
Order number
Unique Device Identifier
CE marking
Important notes/warnings
Medical device
Observe the electronic instructions for use
Switzerland - Authorisation
Fragile, handle with care
Store in a dry place
Temperature limits
Humidity limit
START/STOP
Button
AUTO button day/
night button
Cuff connection
socket
Battery compartment
Batteries
2 LR6 (AA)
Micro USB connection
on the rear Battery compartment cover
Device overview
Action note for the user
Fig. 1
6 7
Symbols on the measurement deviceSymbols on the measurement device
OLED display
START/STOP AUTO/
START/STOP AUTO/
START/STOP button
Sleep mode active
Automatic mode active
AUTO button (DAY/NIGHT BUTTON)
Battery charged
Battery partially charged
Battery empty no further measurement
or data transfer possible
Storage full, 600 measurements,
no further measurements possible
Serial number
Protection against foreign bodies and water:
The IP classification is the degree of protection provided by
enclosures according to IEC 60529. This unit is protected
against solid foreign objects with a diameter of 12 mm and
larger, such as fingers.
This unit is protected against falling dripping water when
the housing is tilted up to 15°.
8
DE
Symbol Funktion/Bedeutung
Systolischer Blutdruck in mmHg
Diastolischer Blutdruck in mmHg
Puls pro Minute
Gerät ist konform mit der europäischen
Medizinprodukterichtlinie.
Gerät darf nicht über den Hausmüll entsorgt werden.
Hersteller
Gebrauchsanweisung lesen
Polarität der Netzgerätanschlussbuchse
SYS
DIA
PUL
0124
Symbole auf dem Blutdruckmessgerät
_boso_medicusX_1711sd.qxd:Layout 1 15.11.2017 13:43 Uhr Seite 8
8
DE
Symbol Funktion/Bedeutung
Systolischer Blutdruck in mmHg
Diastolischer Blutdruck in mmHg
Puls pro Minute
Gerät ist konform mit der europäischen
Medizinprodukterichtlinie.
Gerät darf nicht über den Hausmüll entsorgt werden.
Hersteller
Gebrauchsanweisung lesen
Polarität der Netzgerätanschlussbuchse
SYS
DIA
PUL
0124
Symbole auf dem Blutdruckmessgerät
_boso_medicusX_1711sd.qxd:Layout 1 15.11.2017 13:43 Uhr Seite 8
Read instruction manual
Do not dispose of the appliance
in the household waste .
Defibrillation protected device type BF
Manufacturer
Date of manufacture
Display of the measurement values:
SYS - Systolic blood pressure
DIA - Diastolic blood pressure
PUL - Pulse
mmHg - Unit for blood pressure
/min - Unit for pulse
888
Pulse
Clock display,
if no measurement is taking place
Fault display

8 9
Introduction
Dear customer, we are very pleased that you have decided to purchase
a boso long-term blood pressure monitor.The boso brand stands for the
highest quality and precision. Currently, 96 % of all German general
practitioners, practitioners and internists work with boso blood pressure
monitors in their practice (API study by GfK 01/2016). This device has
passed our strict quality control and is your safe partner for checking
your patients' blood pressure values.
Please read these instructions for use carefully before using the
device for the first time, as correct blood pressure measurement
is only possible if the device is handled correctly.
These instructions for use for the devices of the boso TM series
familiarise you with the use of the ambulatory blood pressure monitor
and the associated accessories. To be able to use all device settings,
measurement protocols as well as all evaluation options of the recorded
blood pressure measurements, you also need the medical software
bosoXD prole manager. For instructions on how to use the software,
please refer to the separate software instructions for use.
Please familiarise yourself with both instructions for use before rst
use. The manufacturer reserves the right to change the information in
these instructions for use without notice. The current version can be
downloaded from the website: https://www.boso.de/downloads
The instruction manual must be kept with the product to have it
available at all times.
In these instructions for use is used for an action of the user.
Non-invasive recording of systolic and diastolic blood pressure values
and pulse rate of individuals over a period of usually 24 hours.
Intended purpose
For help with commissioning, use or maintenance, please contact your
specialist dealer or the manufacturer (contact details on the back cover
of this instruction manual).
These instructions for use must be enclosed with the unit when it is sold.
This blood pressure monitor complies with the current
European regulations and the international standard IEC 80601-2-30:
"Particular requirements for the safety, including essential performance,
of automated non-invasive blood pressure measurement devices".
The use of the device in pregnant women or in pre-eclampsia is not
intended.
If the device is used for medical purposes (in accordance with the
Medical Devices Operator Ordinance), metrological checks must be
carried out at regular intervals (see section Test Instructions).

10 11
If liquid has been spilled on the unit, remove the batteries
immediately and send the unit to the customer service address
(section Warranty Conditions/Customer Service) for inspection.
Watch out for damage to the rechargeable batteries or batteries.
Never use damaged batteries.
Safety information
Notes/safety instructions
Compression or a reduction in the cross-section of the air hose
must be avoided.
Excessively frequent measurements can lead to injuries by
impairing the blood flow.
The cuff must not be applied over wounds as this may cause
further injury.
Make sure that the cuff is not applied to an arm whose arteries
or veins are or have been under medical treatment (e.g. shunt).
For women with a mastectomy, do not apply the cuff to the arm
on the amputated side of the body.
During the measurement, malfunctions may occur in medical
devices that are used simultaneously on the same arm.
The unit has no protection against possible influences from high
frequency (HF) surgical equipment.
Scope of application
Side-effects of the blood pressure
measurement over 24 hours
Petechiae, bleeding or subcutaneous haematomas on the measuring
arm can occur with any blood pressure measurement, even if the cuff
fits correctly.
Patient-dependent risk as a result of treatment with anticoagulants or
patients with coagulation disorders occurs regardless of the type of
meter.Always check if the patient has coagulation disorders or is being
treated with anticoagulants.
The blood pressure monitors of the boso TM series work according
to the oscillometric measuring principle. The device is intended for
24h measurement in the patient's usual environment and is to be
used only under medical supervision and after precise instruction by
doctors or medical professionals. The device is not suitable for infants,
newborns, or for unsupervised use with unconscious patients or patients
with impaired cognitive abilities.

12 13
Medical electrical equipment is subject to special precautions
regarding electromagnetic compatibility and must be installed
and commissioned in accordance with the EMC instructions
section.
Maintenance work on this unit must be carried out by trained and
authorised personnel.
Due to the risk of strangulation by the tube and cuff, the device
must not be within the reach of unsupervised children or used
on unsupervised patients with impaired cognitive abilities or on
patients under anaesthesia.
The device must not be used by children without supervision.
Do not use the device near babies. This can lead to accidents or
damage.
The manufacturer is only responsible for the impact on the safety,
reliability and performance of the device if:
Assembly, extensions, new settings, changes or repairs have been
carried out by persons authorised by him.
The appliance is used in accordance with the instructions for use.
Safety informationSafety information
There is a risk of strangulation with the shoulder strap and cuff
tube.
A patient with impaired cognitive abilities may only use the
device under supervision.
Do not place the shoulder strap and cuff tubing around the
patient's neck.
The cuff hose is always laid under the clothing (also at night).
If the appliance is used with children, this must be done with
special care and under constant supervision.
Instruct the patient to turn off the device, remove the cuff and
notify the doctor if they experience pain, swelling, redness or
numbness in the arm around which the cuff is placed. (It is likely
that the patient may experience mild to moderate discomfort
when having their blood pressure measured.)
The measuring process can be interrupted at any time by pressing
one of the buttons. This deflates the cuff and the device can be
removed.
Instruct the patient to protect the unit from liquid penetration.
In particular, the patient must be advised not to wear the device
while showering.
If the unit has been exposed to moisture or if liquid has entered
during cleaning/use, it must no longer be fitted to patients.
14 15
Selection and connection of the cuff
on boso TM series devices
Cuff selection
Only original cuffs CA91, CA91R, CA92, CA93 and CA94 must
be used.
The cuff must be selected according to the printed arm circumference.
Connecting the cuff
The air connection plug of the cuff tubing is screwed directly into the air
connection socket of the blood pressure monitor (see Fig. 4).
Commissioning
Before you start working with boso TM series devices, you should
charge the batteries supplied. To do this, proceed as described in
section ''Changing and charging the batteries''. Then install the
boso XD profile manager. This software enables the programming
of the blood pressure monitor and the evaluation of the stored
data.
Do not start the machine without putting the cuff on.
The appliance contains small parts; these can cause a choking
hazard if accidentally swallowed by babies.
The performance of the unit may be affected by excessive
temperature, humidity or altitude.
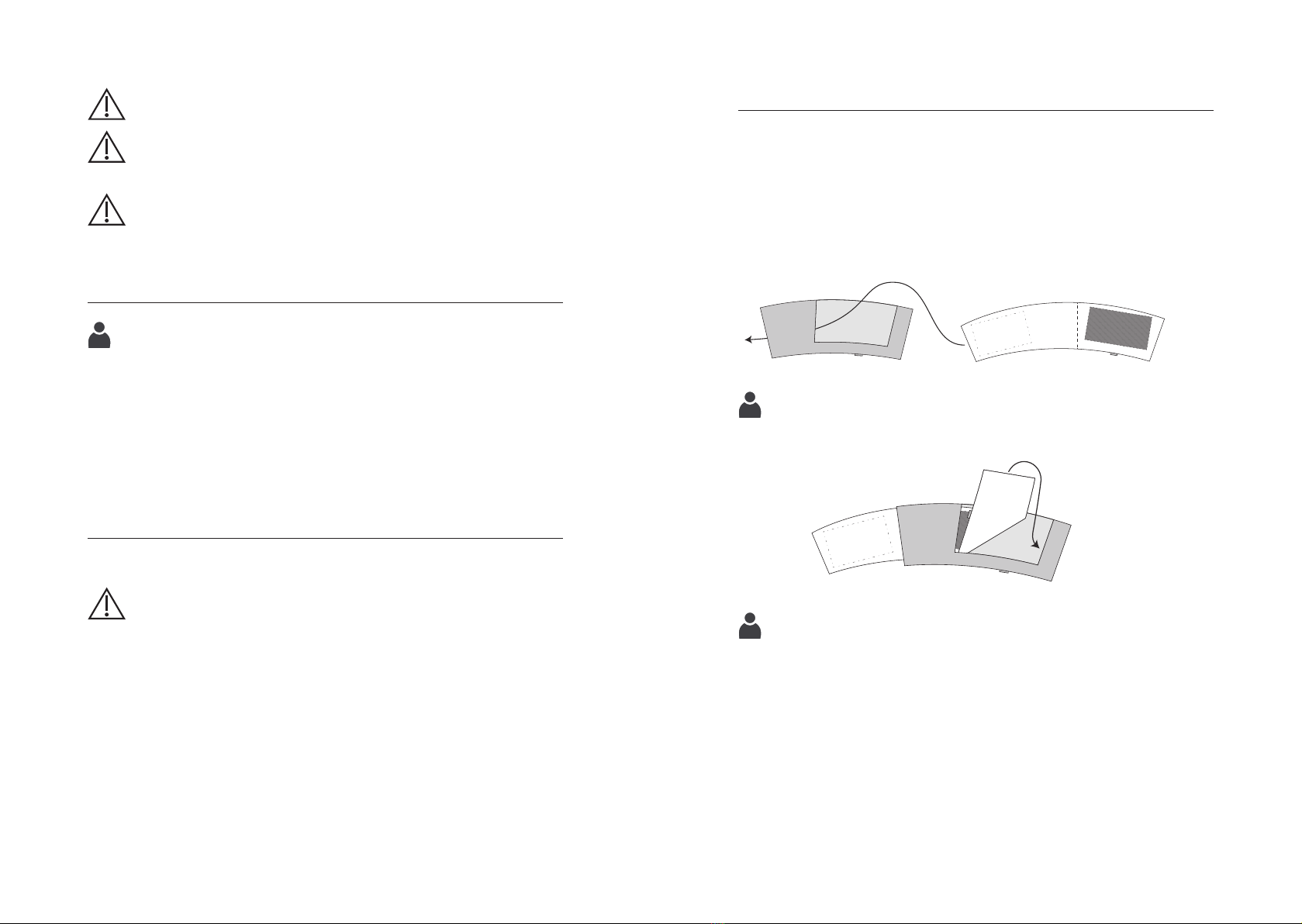
Attachment of the protective covers (optional)
If necessary, you can also use protective covers (see section Accessories)
to protect against soiling.
Put on the protective covers as shown below:
Pull the cuff through the tab of the protective cover.
Care instructions for protective covers:
machine wash at max. 60 °C
boso TM-2430 PC 2
10 St. Schutzbezüge für
Manschette (standard)
Best.Nr. 256-7-400
Manschette durch die Lasche des Schutzbezuges ziehen
Schutzbezug mit den Klettverschlüssen in der Innenseite
an der Manschette befestigen
Pflegehinweis für Schutzbezüge:
Maschinenwaschbar bis max. 60°C
Vor dem Waschvorgang
Klettverschlüsse auf das
danebenliegende
Flauschband klappen
boso TM-2430 PC 2
10 St. Schutzbezüge für
Manschette (standard)
Best.Nr. 256-7-400
Manschette durch die Lasche des Schutzbezuges ziehen
Schutzbezug mit den Klettverschlüssen in der Innenseite
an der Manschette befestigen
Pflegehinweis für Schutzbezüge:
Maschinenwaschbar bis max. 60°C
Vor dem Waschvorgang
Klettverschlüsse auf das
danebenliegende
Flauschband klappen
boso TM-2430 PC 2
10 St. Schutzbezüge für
Manschette (standard)
Best.Nr. 256-7-400
Manschette durch die Lasche des Schutzbezuges ziehen
Schutzbezug mit den Klettverschlüssen in der Innenseite
an der Manschette befestigen
Pflegehinweis für Schutzbezüge:
Maschinenwaschbar bis max. 60°C
Vor dem Waschvorgang
Klettverschlüsse auf das
danebenliegende
Flauschband klappen
boso TM-2430 PC 2
10 St. Schutzbezüge für
Manschette (standard)
Best.Nr. 256-7-400
Manschette durch die Lasche des Schutzbezuges ziehen
Schutzbezug mit den Klettverschlüssen in der Innenseite
an der Manschette befestigen
Pflegehinweis für Schutzbezüge:
Maschinenwaschbar bis max. 60°C
Vor dem Waschvorgang
Klettverschlüsse auf das
danebenliegende
Flauschband klappen
Protective cover Cuff
Attach the protective cover to the cuff with the Velcro fasteners
on the inside.
Fig. 2
Fig. 3
16 17
Cuff connection
socket boso TM
series
Bag
Air connection
plug
White
marking
2–3 cm
Tape
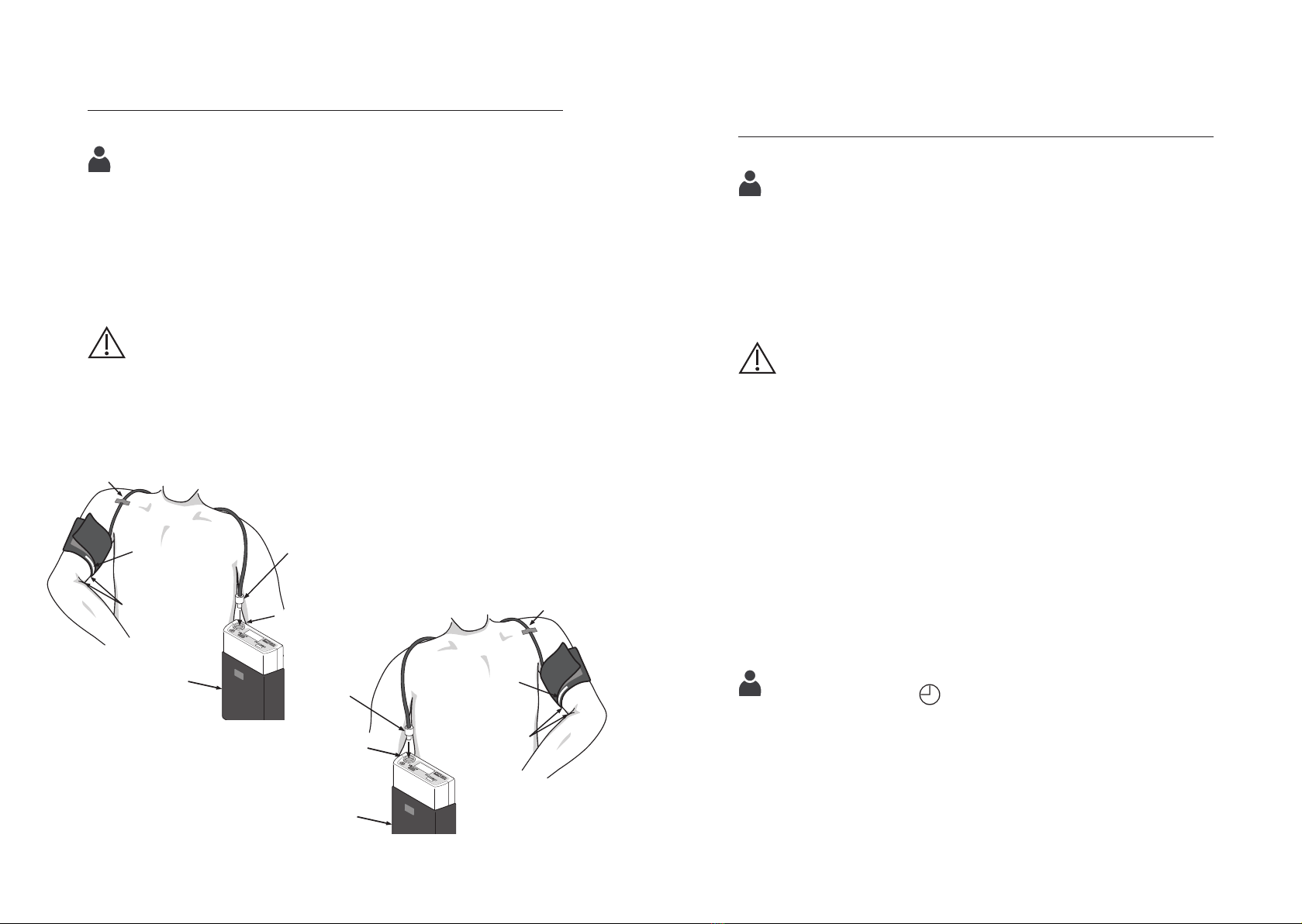
Attaching the cuff
Fig. 4
Cuff connection
socket boso TM
series
Bag
Air connection
plug
White marking
2–3 cm
Tape
Place the cuff on the unclothed upper arm so that the white
marking lies on the arteria brachialis. For most people, blood
pressure is higher on the left arm, so blood pressure is measured
on the left arm. If the blood pressure is higher on the right arm,
it should be measured on the right arm.
The cuff should be approx. 2-3 cm above the crook of the elbow.
The cuff should not be too tight, about two fingers should still fit
between the arm and the cuff.
After the end of the measurement, the blood circulation must
not be impaired by the cuff. Place the cuff tube over the shoulder
(see fig. 4). Fix the cuff tube to the shoulder with tape. The blood
pressure monitor is carried in the pouch either on an existing belt
or with the included carrying strap.
After the cuff has been properly put on, a test measurement
can be triggered on the boso TM series device by means of the
START/STOP button (measurement is only displayed if the device
has been programmed accordingly). If the display is off, activate
it by pressing any button. If this measurement is successful, the
automatic interval function (see below) can be started.The sample
measurement is included in the evaluation.
Please note that the oscillometric measurement method can lead
to measurement inaccuracies in some types of patients. Persons
with cardiac arrhythmia, arteriosclerosis, circulatory disorders,
diabetes or pacemaker wearers should have a comparative
measurement taken with an auscultatory device before starting
the measurement. This also applies to women during pregnancy.
Carrying out measurements
with boso TM series devices
External disturbing influences,such as movements of the measuring arm,
disturbing vibrations, e.g. due to driving or the use of public transport
during the measurement, can lead to incorrect measurements. For this
reason, the record kept by the patient must be viewed and included in
the evaluation of the measurement results.
Starting the automatic interval
To start the automatic interval, press and hold the black
AUTO button until " " appears in the blood pressure monitor
display and is acknowledged by a short signal tone (after approx.
5 seconds).

18 19
If the device is operated in “Sleep button" mode, the patient must
press the black AUTO button before going to sleep. The display shows
"" for automatic and " " for sleep mode. After getting up, the
black AUTO button must be pressed again. The " " in the display
disappears.
Automatic adjustment of the pump-up level
(only in automatic interval mode)
The boso TM series device automatically pumps up to the required
pressure level. If this inflation height is not sufficient, the unit
automatically inflates again approx. 60 mmHg above the original
inflation level.
Limiting the maximum pumping level
The device of the boso TM series has the option of limiting the inflation
height. For the corresponding procedure, please refer to the boso XD
profile manager instructions for use.
Performing a manual measurement
A manual measurement can be started by the patient at any time in
addition to the automatic measurements.This can be sensible following
physical or emotional stress. To do this, activate the display, then press
the white START/STOP button.
Aborting measurements
To cancel the measurement, the white START/STOP button on the
boso TM series must be pressed.
If the measurement is to be taken at a later time, a manual
measurement can be started at any time using the white START/
STOP button.
Ending the measurement and
transferring the measurement data
As soon as the device is removed from the patient after the 24-hour
measurement has been completed, the automatic function must
be switched off. To do this, press and hold the black AUTO button
until the " " disappears from the blood pressure monitor display
(approx. 5 seconds).
Then connect the boso TM series device to the computer using the USB
connection cable.Transfer the data according to the instructions for use
boso XD profile manager.
After transferring the measured data, it his highly suggested to delete
the data memory.
20 21
Changing the batteries
We recommend changing the used battery pack after each 24-hour
measurement and replacing it with the freshly charged battery pack.
To prevent data loss, the data stored in the boso TM series device is
buffered by an internal battery. This battery is automatically charged
via the battery pack.With a fully charged battery pack, the data remain
stored for approx. 10 days. To fully charge the battery when using the
unit for the first time, switch on the unit with fully charged batteries for
approx. 24 hours.
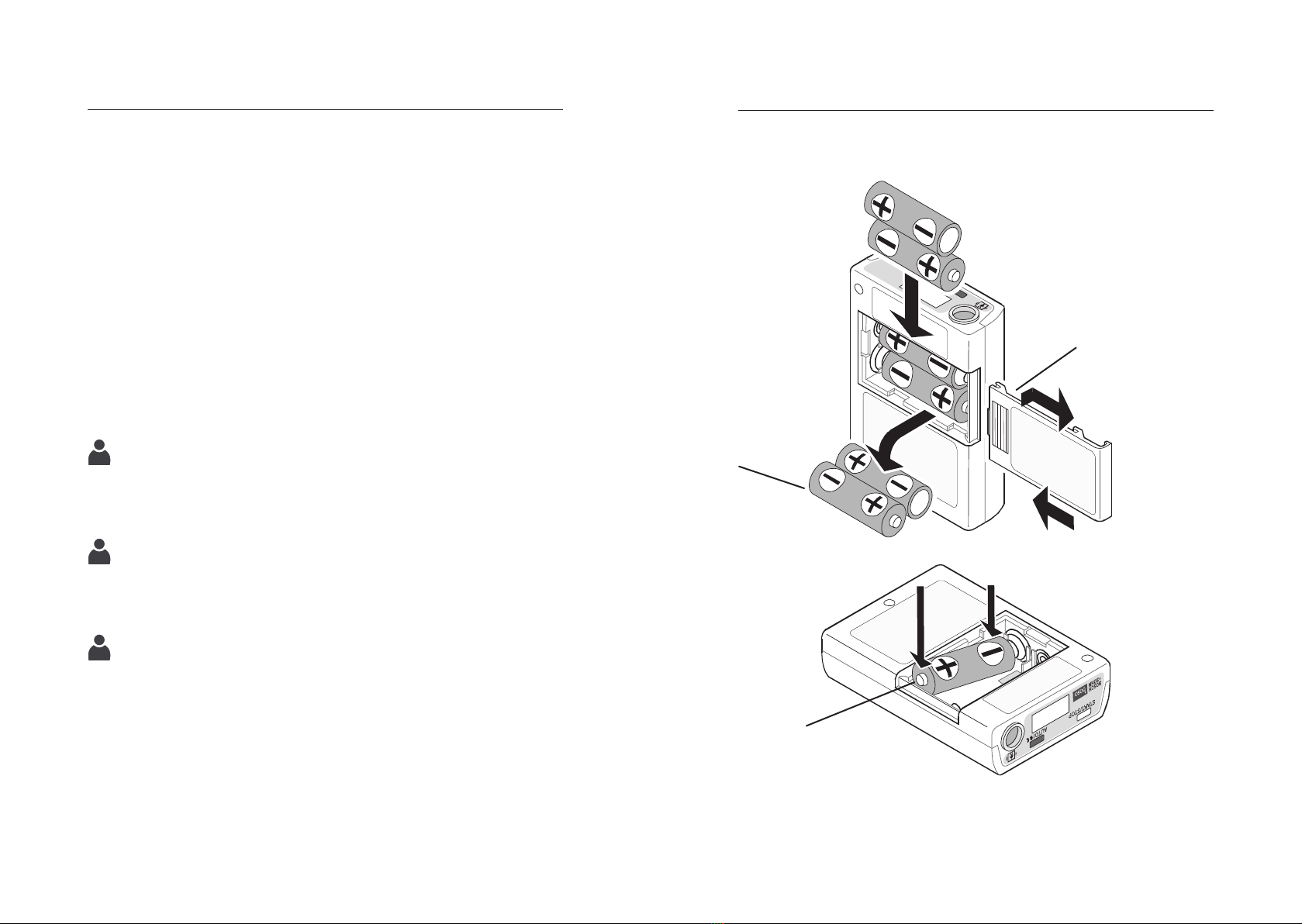
To change the batteries, proceed as follows (see fig. 5):
Open the battery compartment cover (step 1)
Remove the empty batteries (step 2) and reinsert the charged
batteries (step 3) (note polarity!) (Step 4))
Close the battery compartment cover (Step 5)
Changing the batteries
Fig. 5
Insertchargedbatteries
Step 2
Battery
compartment cover
Step 1
Step 3
Step 4
Empty batteries
Step 5

22 23
Charging the batteries
Insert the rechargeable batteries into the charger and plug it into a
power socket. If the blue LED lights up, the battery packs are being
charged.The charging process takes roughly 11 hours for a fully charged
battery pack. After 13 hours, the charger automatically switches off the
charging process.
Important note regarding battery charging
To ensure proper functioning of the boso TM series device for more than
24 hours, use only rechargeable batteries with the following ratings:
min. 1900 mAh; 1.2 V; NiMH
or batteries (Type AA 1.5 V).
In addition to the two rechargeable batteries required for the power
supply, the device of the bosoTM series also contains an internal battery
to save the programme setting in the device.
To avoid loss of programming and stored readings when the internal
battery is discharged, please observe the following procedure:
If the batteries are short-circuited, they may become hot and cause
burns and scorching damage to the unit.
Do not touch the batteries and the patient at the same time.
Insert charged batteries into the unit even when it is not in use.
The state of charge of the internal battery is thus constantly
kept at a high level. If the power supply to the internal battery is
interrupted, the settings of the boso TM series device will be lost
after approx. 10 days.
Before placing the unit on a patient, please replace the batteries
in the unit with a set of freshly charged batteries.
Longer storage of the device
If the unit will not be used for a long time (4 weeks or more), remove
the batteries to prevent possible damage due to leakage.
Before the device is then attached to a patient again,the internal battery
must be charged and the device reprogrammed.
Insert freshly charged batteries.
Please leave the battery packs in the device for two hours.
The internal battery is recharged during this time.
Reprogram the device.
Before attaching the device to a patient, replace the batteries with
a set of freshly charged ones.

24 25
Error messages
Error
code
Cause Remedy
0:00 Time sets to 0:00 when
battery is replaced
Device must be
reprogrammed.
E03
E90
Zero point adjustment not
possible
Deflate cuff completely.
E04 Empty battery Charge or replace
batteries.
E05 Leakage Disconnect the cuff from
the device and reconnect.
If the error occurs
repeatedly, contact your
sales partner.
E06 Pressure above 299 mmHg The arm must be kept still
during measurement.
E07 User abort via START/STOP
button
E08
E10
No or non-interpretable
oscillations
The arm must be kept still
during measurement.
E09 Error of the activity sensor Remove and re-insert the
battery packs.
Error
code
Cause Remedy
E20 Pulse < 30 or > 200
Check the position and fit
of the cuff.
E21
E22
No interpretable oscillations
in the area of diastole (E21)
or systole (E22)
E23 Systole-diastole < 10 or
> 150 mmHg
E30 Measuring time longer than
180 seconds
Contact your sales
partner.
E31 Deflation longer than
90 seconds
Contact your sales
partner.
E48 Pulse cannot be measured The arm must be kept still
during measurement.
E52 Storage error Contact your sales
partner.
E91 Pressure inside the cuff too
high / maximum pressure
set too low
Set a higher maximum
pressure. The arm must
be kept still during
measurement.
Error messages

26 27
After use
Cleaning and disinfection
To clean the boso TM series device and the cuff, please use a soft
cloth that may be moistened with soapy water.
For the protective covers: machine wash at max. 60 °C.
Never use solvents, benzine, spirits or abrasive cleaners for
cleaning!
Disinfection:
For disinfection by wiping (exposure time at least 5 minutes) the
device, we recommend the disinfectant Antifect Liquid (Schülke &
Mayr).We recommend using spray disinfection for disinfecting the
cuff. Especially if the device is used by several patients, make sure
that the cuff is cleaned and disinfected regularly.
Disposal information
Usedbatteriesandrechargeablebatteriesmustnotbedisposed
of in household waste.You can take them to a collection point
for used batteries or to hazardous waste. Please contact your
municipality for more information.
1) Purpose
Based on the EU Directive 2012/19/EU, the German implementation of
the ElektroG was revised in 2021. The amended ElektroG3 came into
force on 01.01.2022. The background to this is to steadily improve the
collection rates of e-waste and to achieve a rate of > 65 %. In this
document we inform you about the return option we have created for
your waste electrical equipment (WEEE) from the commercial sector.
2) Manufacturer's declaration on the return option
For commercially used appliances, a collection can take place at the end
of the life cycle via our recycling partner (see point 3). For this purpose,
a notification must be sent to our return partner or to "Bosch+ Sohn
GmbH u. CO. KG", stating the articles and their number. The customer
then receives an offer from our recycling partner for coordinated
collection at the point of generation.The customer is free to opt for this
collection or to take the WEEE to his own disposal system and to comply
with the corresponding obligations.
3) Authorised recycling partner
The authorised recycling company for the company "Bosch+Sohn
GmbH u. CO. KG" is:
WEEE Return GmbH
Lahnstraße 31
12055 Berlin
Customer information on the recycling of
commercial waste electrical equipment

28 29
Obligation to report incidents
A serious incident shall be reported to the manufacturer and to the
responsible authority of the Member State in which the user and/or
patient is established.
A "serious incident" means an incident that directly or indirectly had,
could have had, or may have had any of the following consequences:
the death of a patient,user,or other person; the temporary or permanent
serious deterioration of the health of a patient, user, or other person; a
serious public health hazard. Please send reports of serious incidents to:
E-Mail: vigilanz@boso.de
Fax: +49 (0) 74 77 92 75-56
4) Contact details for the return option
A return can be registered by telephone or by e-mail. The following
options are available to the waste owner:
Phone: +49 (0) 74 77 92 75-0
E-Mail: zentrale@boso.de
Warranty conditions / customer service
This product is covered by a 2-year factory warranty from the date of
purchase.
The date of purchase must be proven by invoice. Within the warranty
period, defects resulting from material or manufacturing faults will be
repaired free of charge. Guarantee services do not extend the guarantee
period for the entire appliance, but only for the replaced components.
The warranty does not cover wear and tear (e.g. cuff), transport damage
or any damage caused by improper handling (e.g. failure to follow
the instructions for use) or by tampering by unauthorised persons.
The guarantee does not give rise to any claims for damages against us.
The buyer's statutory claims for defects pursuant to Art. 437 of the
German Civil Code (BGB) shall not be restricted.
In the event of a claim under the warranty,the unit must be sent together
with the original proof of purchase to:
BOSCH + SOHN GmbH u. Co. KG
Bahnhofstr. 64, 72417 Jungingen, Germany
Maintenance work on this unit must be carried out by trained and
authorised personnel.
Theunitmustnotbemodifiedwithoutthepermissionofthemanufacturer.

30 31
Accessories
Pleaseuseonlytheaccessoriesrecommendedbythemanufacturer.
Cuffs
Size M CA91 20 - 31 cm 259-4-400
Size M (right) CA91R 20 - 31 cm 259-4-440
Size L CA92 28 - 38 cm 259-4-410
Size XL CA94 36 - 50 cm 259-4-430
Size S CA93 15 - 22 cm 259-4-420
Protective covers (10 pieces)
Size M left and right 259-7-400
Size L 259-7-410
Size XL 259-7-430
Size S 259-7-420
5x size M and 5x size L 259-7-405
Other accessories
Charger 535-7-130
NiMh batteries (2 pieces, Mignon) 535-7-131
Waist bag with carrying strap 515-7-116
Technical specifications
Product: Blood pressure measuring device
for 24-hour measurement
Type designation: See equipment labelling
Rated voltage: 2x 1.5 V DC or 2x 1.2 V DC
Power supply: 2x NiMh batteries (Mignon)
Measuring range: Systole: 60 - 280 mmHg
Diastole: 30 - 160 mmHg
Pulse: 30 - 200 pulse/min
Maximum deviation of the cuff
pressure:
±3 mmHg or 2 % of reading
(whichever is greater)
Maximum deviation of the
pulse reading:
±5 %
Measurement storage: 600 measurements
Operating conditions: +10 °C to +40 °C
rel. humidity 30 - 85 %
(non-condensing)
Air pressure 700 - 1060 hPa
Storage conditions: +20 °C to +60 °C
10 - 95 % rel. humidity
Air pressure 700 - 1060 hPa
Weight: 135 grammes without batteries
32 33
Technical specifications Test instruction for metrological checks
A) Functional check
A functional check of the unit can only be carried out on humans or
with a suitable simulator.
B) Checking for tightness of the pressure circuit and deviation
of the pressure indication
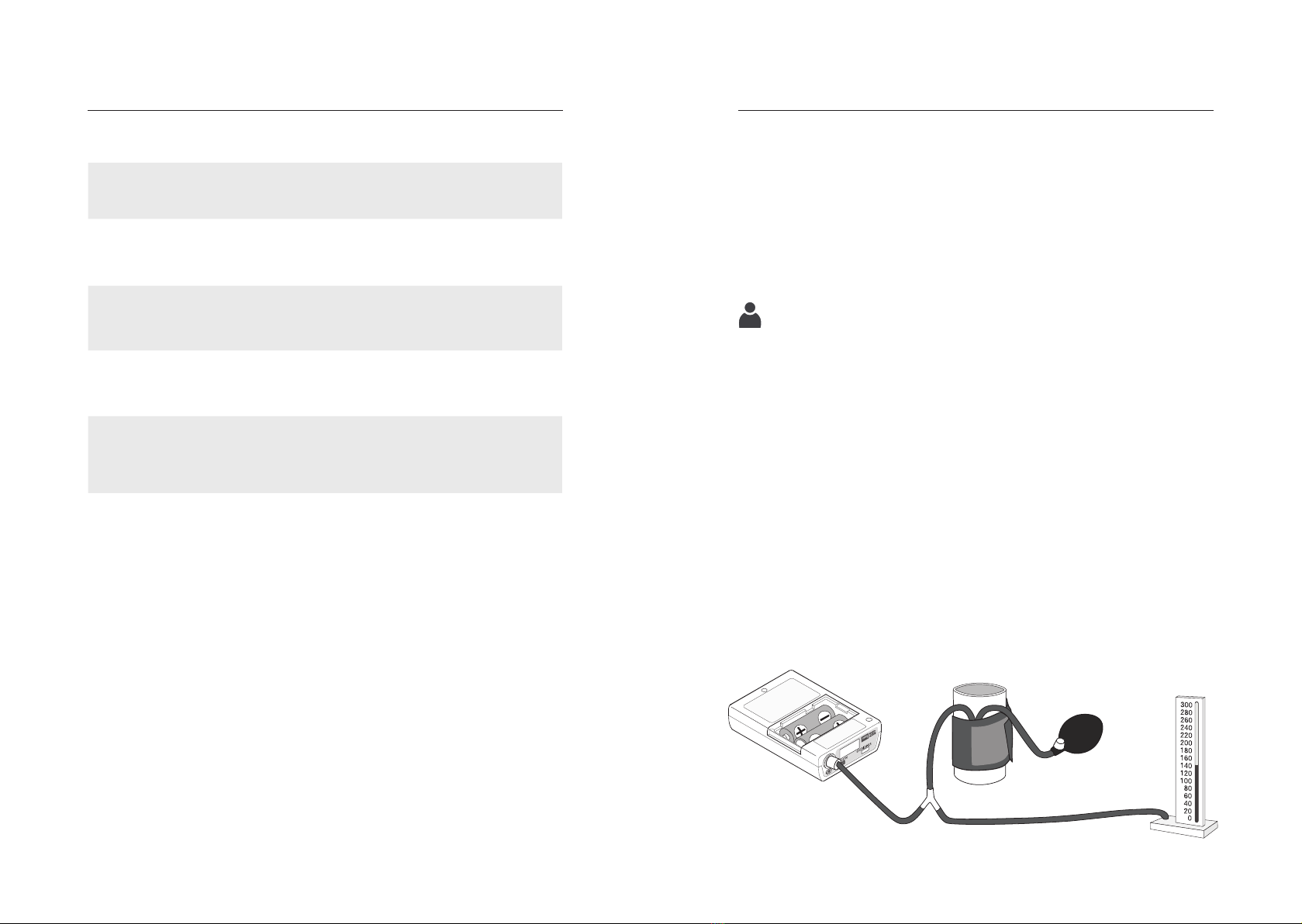
Remove the batteries. Then create a test setup as shown in Fig. 6.
Immediately after reinserting the batteries, press and hold the
white START/STOP button. The white START/STOP button must be
held down until a "0" appears in the display of the boso TM series
device.
Then carry out the test for deviation of the pressure indication
and tightness of the pressure circuit (observe the setting time of
the cuff - at least 30 seconds) in the usual way. To return to the
measuring mode after the test has been completed, the batteries
must be removed again and reinserted.
C) Securing
For securing, the housing halves (upper and lower part) are connected
with a securing tag.
Fig. 6
START/STOP
button
Release valve
Pressure test device
Pressure ball
Dimensions (W x H x D): 66 mm x 25 mm x 95 mm
Typical battery life: 1000 charging cycles (depending on
pump-up level + frequency of use)
Expected service life of
the unit: 10 years
Expected service life of
the cuff: 10,000 measuring cycles
Clinical test: The measurement accuracy meets
the requirements of ISO 81060-2

34 35
EMC information
Medical electrical equipment is subject to special precautions regarding EMC and must be installed and
commissioned in accordance with the guidelines given below.
Portable and mobile HF devices (e.g. mobile phones) can affect medical electrical equipment.
The use of third-party accessories (not original boso parts) may result in increased emissions or reduced
immunity of the device.
Guidelines and manufacturer's declaration - Electromagnetic emissions
The boso blood pressure monitor is intended for operation in the electromagnetic environment specified below. The
customer or the user of the boso blood pressure monitor should ensure that it is used in such an environment.
Guidelines and manufacturer's declaration - Electromagnetic interference immunity
The boso blood pressure monitor is intended for operation in the electromagnetic environment specified below.The customer
or the user of the boso blood pressure monitor should ensure that it is used in such an environment.
NOTE: UTis the AC mains voltage before the test level is applied.
EMC information
Guidelines and manufacturer's declaration - Electromagnetic emissions
The boso blood pressure monitor is intended for operation in the electromagnetic environment specified below.
The customer or the user of the boso blood pressure monitor should ensure that it is used in such an environment.
With P as the nominal power of the transmitter in watts (W) according to the transmitter manufacturer's
specifications and d as the recommended protective distance in metres (m). The field strength of stationary
radio transmitters is lower than the compliance levelbat all frequencies according to an on-site investigationa.
Interference is possible in the vicinity of units with this symbol.
NOTE 1: For 80 MHz and 800 MHz, the higher value applies.
NOTE 2: These guidelines may not apply in all situations. The propagation of electromagnetic waves is influenced by
absorption and reflection from buildings, objects and people.
aThe field strength of stationary transmitters, such as base stations of radio telephones and land mobile services,
amateur stations, AM and FM radio and television transmitters, cannot theoretically be predicted exactly. To determine
the electromagnetic environment due to stationary HF transmitters, a site survey is recommended. If the determined field
strength at the location of the boso blood pressure monitor exceeds the compliance level specified above, the boso blood
pressure monitor must be observed with regard to its normal operation at any application location. If unusual performance
characteristics are observed, it may be necessary to take additional measures, such as reorienting or relocating the boso
blood pressure monitor. bOver the frequency range from 150 kHz to 80 MHz, the field strength is less than 3 V/m.
Recommended safe distances
between portable and mobile HF communication devices and the boso blood pressure monitor.The boso blood pressure monitor
is intended for operation in an electromagnetic environment in which radiated HF disturbances are controlled. The customer or
the user of the boso blood pressure measuring device can help prevent electromagnetic interference by maintaining minimum
distances between portable and mobile HF communications equipment (transmitters) and the boso blood pressure measuring
device as recommended below according to the maximum output power of the communications equipment.
For transmitters whose rated power is not specified in the above table, the distance can be determined using the equation
associated with the respective column, where P is the rated power of the transmitter in watts (W) as specified by the
transmitter manufacturer.
NOTE 1: An additional factor of 10/3 was used to calculate the recommended separation distance of transmitters in
the frequency range from 80 MHz to 2.5 GHz to reduce the likelihood that a mobile/portable communication device
inadvertently introduced into the patient area would cause interference.
NOTE 2: These guidelines may not apply in all situations. The propagation of electromagnetic waves is influenced by
absorption and reflection from buildings, objects and people.
Emission measurements Compliance Electromagnetic Environment Guidelines
HF emissions according to CISPR 11 Group 1 The boso blood pressure monitor uses HF energy
exclusively for its internal function. Therefore, its HF
emission is very low and it is unlikely to interfere with
nearby electronic equipment.
HF emissions according to CISPR 11 Class B The boso blood pressure monitor is intended for use in
all establishments, including domestic establishments
and those directly connected to a public supply network
which also supplies buildings used for domestic
purposes.
Harmonics according to IEC 61000-3-2 not applicable
Voltage fluctuations/ flicker according
to IEC 61000-3-3
Interference immunity tests IEC 60601-test level Compliance level Electromagnetic
Environment -
Guidelines
Static electricity discharge (ESD)
according to IEC 61000-4-2
±6 kV contact discharge
±8 kV air discharge
±6 kV contact discharge
±8 kV air discharge
Floors should be wood,
concrete or ceramic tiles.
If the floor is covered
with synthetic material,
the relative humidity
must be at least 30 %.
Fast transient electrical
disturbance/bursts according to
IEC 61000-4-4
±2 kV mains cables
±1 kV for input and output
lines
Not applicable
Surges according to IEC
61000-4-5
±1 kV push-pull voltage
±2 kV common-mode
voltage
Not applicable
Voltage dips, short-time
interruptions and fluctuations
of the supply voltage according
to IEC 61000-4-11
< 5 % UTfor 1/2 period
(> 95 % dip)
40 % UTfor 5 periods
(65 % dip)
70 % UTfor 25 periods
(30 % dip)
< 5 % UTfor 5 s
(> 95 % dip)
Not applicable
Magnetic field at the supply
frequency (50/60 Hz) according
to IEC 61000-4-8
3 A/m 3 A/m
Interference immunity
tests
IEC 60601-test
level
Compliance
level
el-magn. Environment - Guidelines
recommended safe distance
Portable and mobile radios are used at
no closer distance from the boso blood
pressure monitor, including the leads, than
the recommended protective distance
calculated using the equation appropriate
to the transmitting frequency:
d = 1.2 P
Conducted HF disturbances
according to IEC 61000-4-6
3 Veff
150 kHz - 80 MHz
3 Vef
radiated HF disturbances
according to IEC 61000-4-3
3 V/m
80 kHz - 2.5 GHz
3 V/m d = 1.2 P for 80 MHz - 800 MHz
d = 2.3 P for 800 MHz - 2.5 GHz
Rated power of the transmitter Protective distance according to transmission frequency m
W150 kHz to 80 MHz
d = 1.2 P
80 MHz to 800 MHz
d = 1.2 P
800 MHz to 2.5 GHz
d = 2.3 P
0.01 0.12 0.12 0.23
0.01 0.38 0.38 0.73
11.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23

36
BOSCH + SOHN GmbH u.Co. KG
Bahnhofstraße 64 |72417 Jungingen, Germany |T +49 (0) 7477 9275-0 |F +49(0)7477 1021
E zentrale@boso.de |www.boso.de
11/2022 | Errors and omissions excepted.
Table of contents
Other Bosch+Sohn Blood Pressure Monitor manuals

Bosch+Sohn
Bosch+Sohn Boso-medicus exclusive User manual

Bosch+Sohn
Bosch+Sohn boso TM-2450 User manual
Bosch+Sohn
Bosch+Sohn medicus family 4 User manual

Bosch+Sohn
Bosch+Sohn boso medicus system User manual

Bosch+Sohn
Bosch+Sohn Boso medicus prestige S User manual

Bosch+Sohn
Bosch+Sohn Boso Nova S User manual
Bosch+Sohn
Bosch+Sohn BoSo Medicus Smart User manual