&
&
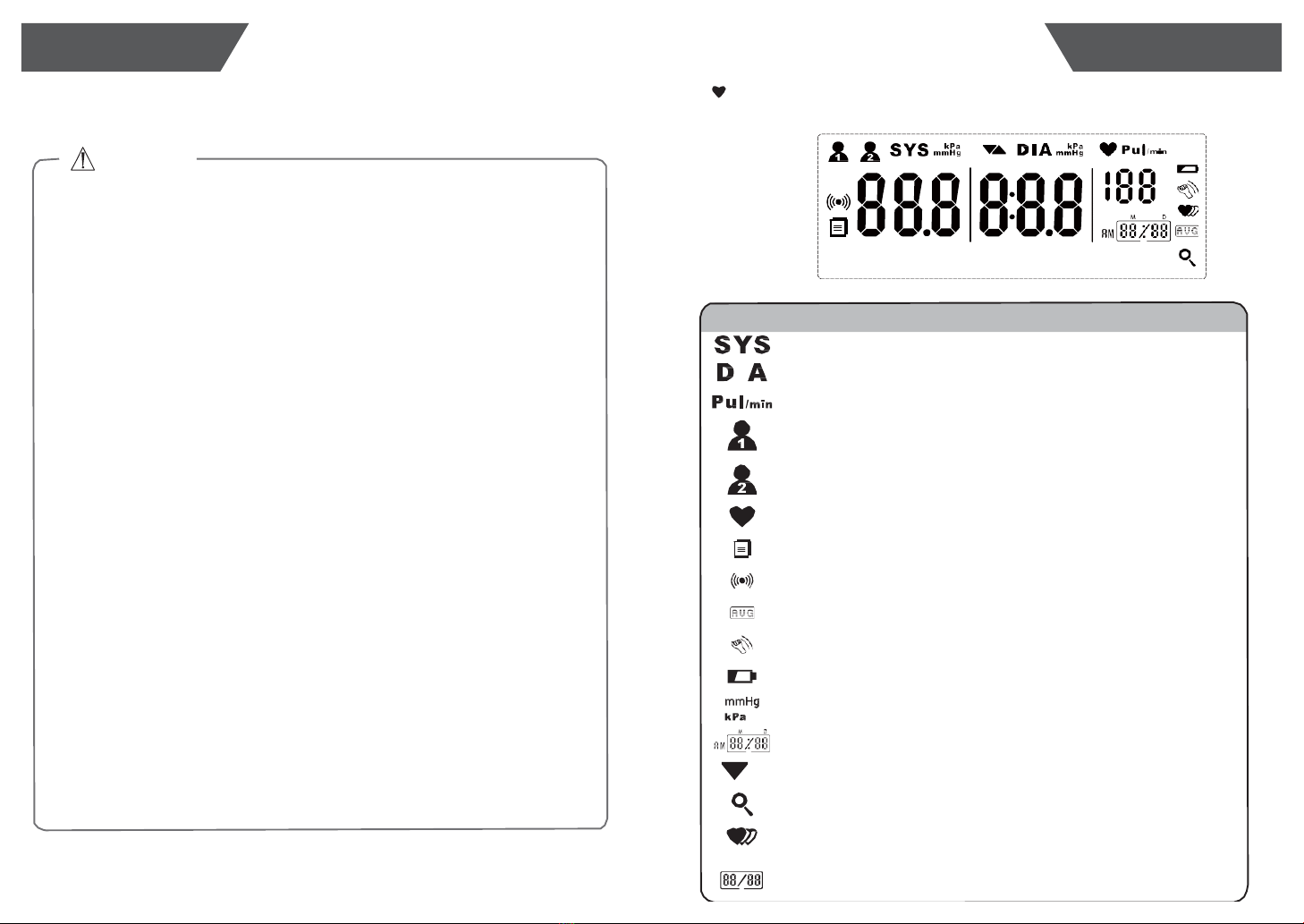
LCD Display Signal
CAUTION
This device is intended for adult use only.
This device is intended for no-invasive measuring and monitoring of arterial blood pressure.
It is not intended for use on extremities other than the arm or for functions other than obtaining a
blood pressure measurement.
Do not confuse self-monitoring with self-diagnosis. This unit allows you to monitor your blood
pressure.Do not begin or end medical treatment without asking a physician for treatment advice.
If you are taking medication,consult your physician to determine the most appropriate time to
measure your blood pressure. Never change a prescribed medication without consulting your
Physician.
When the device was used to measure patients who have common arrhythmias such as atrial or
ventricular premature beats or atrial fibrillation, the best result may occur with deviation. Please
consult your physician about the result.
If the cuff pressure exceeds 40 kPa (300 mmHg), the unit will automatically deflate. Should the
cuff not deflate when pressures exceeds 40 kPa (300 mmHg), detach the cuff from the arm and
press the corresponding user button to stop inflation.
The equipment is not AP/APG equipment and not suitable for use in the presence of a flammable
anesthetic mixture with air of with oxygen or nitrous oxide.
The operator shall not touch output of batteries /adapter and the patient simultaneously.
To avoid measurement errors, please avoid the condition of strong electromagnetic field radiated
interference signal or electrical fast transient/burst signal.
The user must check that the equipment functions safely and see that it is in proper working
condition before being used.
This device is contraindicated for any female who may be suspected of, or is pregnant. Besides
providing inaccurate readings, the effects of this device on the fetus are unknown.
Manufacturer will make available on request circuit diagrams, component parts list etc.
This unit is not suitable for continuous monitoring during medical emergencies or operations.
Otherwise, the patient’s arm and fingers will become anaesthetic, swollen and even purple due to
a lack of blood.
Please use the device under the environment which was provided in the user manual. Otherwise,
the performance and lifetime of the device will be impacted and reduced.
During use, the patient will be in contact with the cuff. The materials of the cuff have been tested
and found to comply with requirements of ISO 10993-5:2009 and ISO 10993-10:2010. It will not
cause any potential sensization or irritation reaction.
Please use ACCESSORIES and detachable partes specified/ authorised by MANUFACTURE.
Otherwise, it may cause damage to the unit or danger to the user/patients.
The device doesn’t need to be calibrated within the two years of reliable service.
Please dispose of ACCESSORIES, detachable parts, and the ME EQUIPMENT according to the
local guidelines.
If you have any problems with this device, such as setting up, maintaining or using, please
contact the SERVICE PERSONNEL of iChoice. Don’t open or repair the device by yourself.
Please report to iChoice if any unexpected operation or events occur.
Please use the soft cloth to clean the whole unit. Don’t use any abrasive or volatile cleaners.
4 5