Boston Scientific (Master Brand DFUTemplate 8.2677in x 11.6929in A4, 92238519A), eOP MANUAL, MB, Rezūm, en, 50909085-01A
Black (K) ∆E ≤5.0
DANGER
Do not take or use the device in locations where combustible
anesthetics or flammable gases are used or in high-pressure
oxygen rooms or inside oxygen tents.
After use, dispose of product and packaging in accordance
with hospital, administrative and/or local government policy.
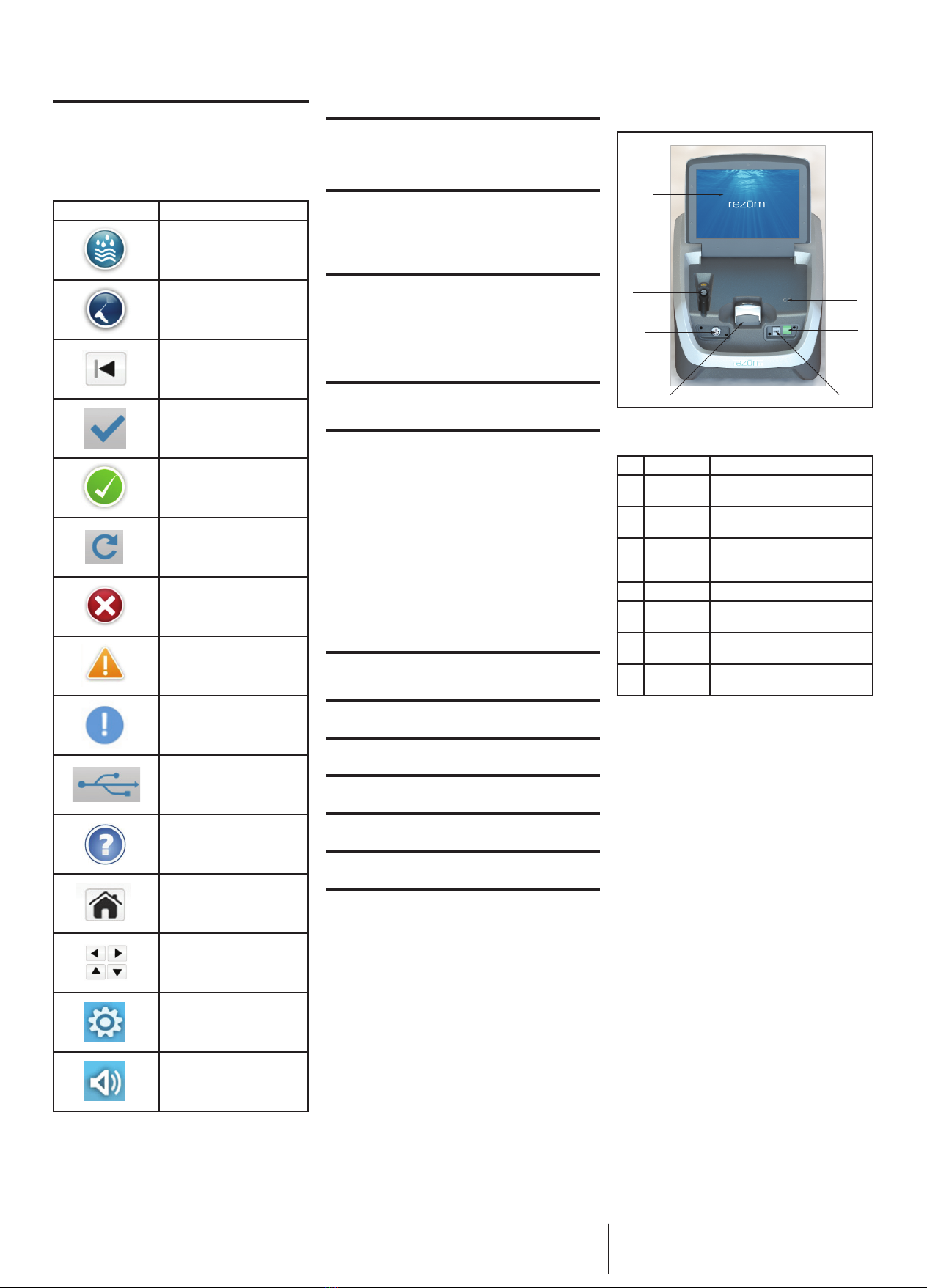
DEVICE DESCRIPTION
The Rez
ū
m™ Generator is designed to heat the RF coil within
the hand-held Delivery Device rapidly. The device uses radio
frequency (RF) energy to heat water into vapor outside the body.
The vapor is injected into the tissue and rapidly disperses
through the interstitial spaces between the tissue cells. The
vapor begins to cool and condenses immediately on contact with
tissue. The stored heat energy is released, gently denaturing the
cell membranes and causing instantaneous cell death.
The denatured tissue is absorbed by the body over time. The
vapor condensation process also causes a rapid collapse of
blood vessels in the ablation treatment zone, resulting in a
bloodless procedure.
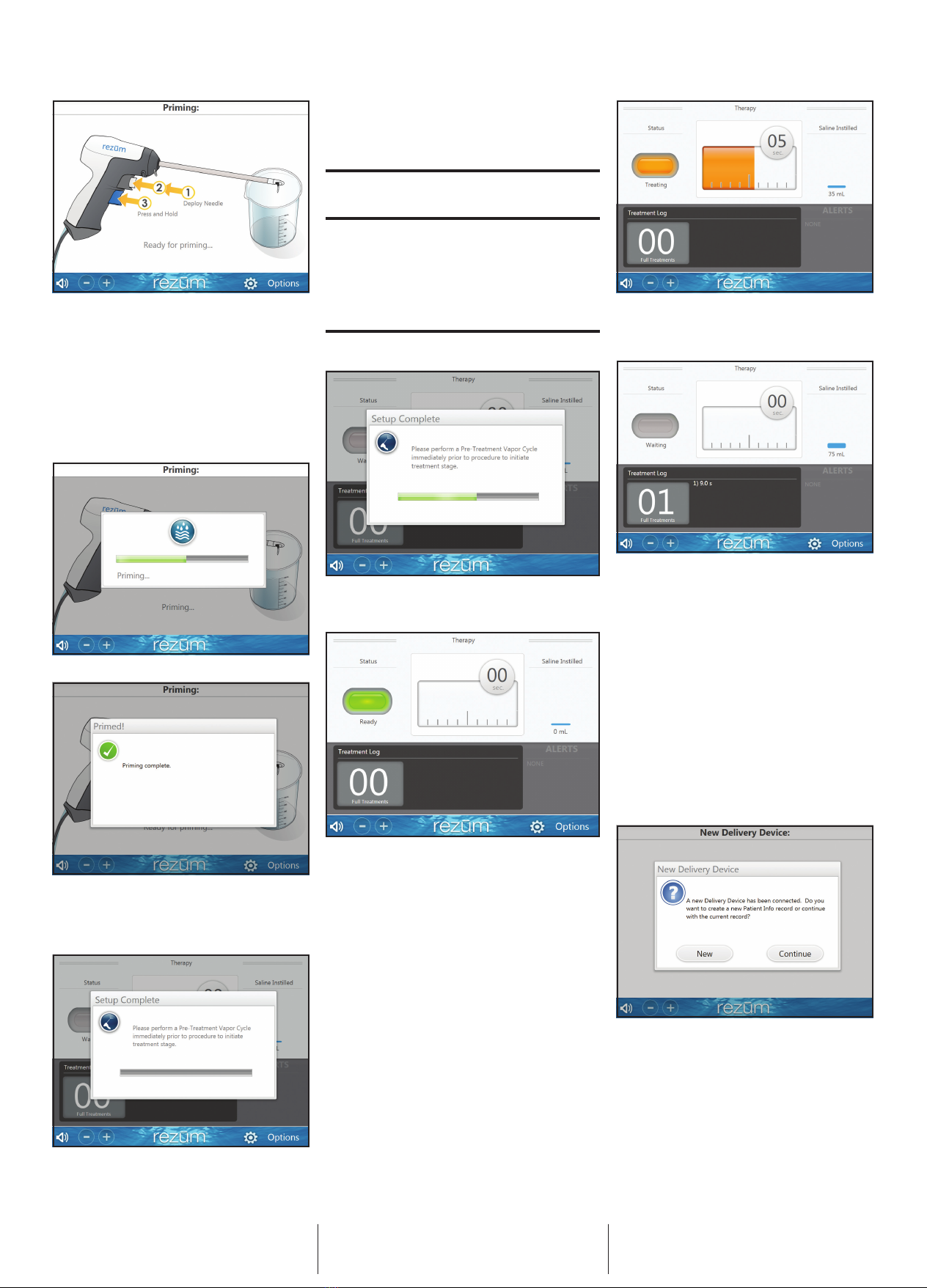
The generator rapidly heats and converts sterile water into
nearly pure vapor at slightly above 100°C. The generator
delivers this thermal energy in the form of vapor through
precise vapor emitter openings at the tip of the Vapor Emitter
Needle. The rate and time over which the thermal energy in
the form of vapor is delivered is monitored and regulated by
the generator.
INTENDED USE/INDICATIONS FOR USE
The Rez
ū
m Generator is intended to be used with the Rez
ū
m
Delivery Device Kit, Model D2201 only. Refer to the Directions
for Use for the Rez
ū
m Delivery Device Kit for the Indications for
Use/Intended Use.
CONTRAINDICATIONS
Refer to the Directions for Use for the Rez
ū
m Delivery Device
Kit for the Contraindications.
WARNINGS
A protective ground connection by way of the grounding
conductor in the power cord is essential for safe operation.
To avoid electrical shock, plug the power cord into a properly
wired receptacle, use only the power cord supplied with the
generator, and make sure the power cord is in good condition.
After visual inspection, if the generator is damaged or a
message is indicated to not use the generator, please contact
Boston Scientific Technical Service and take the generator out
of service.
Before conducting routine care, turn the power off and unplug
the power cord from the outlet to prevent electric shock.
Do not modify this equipment.
Carefully read and understand all instructions, indications,
warnings, and cautions in this operator’s manual prior to
using the Rez
ū
m Generator. Failure to do so could result in
compromised patient safety, patient complications and/or
insufficient treatment.
Do not connect a grounding wire from a grounding stud to a gas
pipe or water pipe.
Do not connect to an electrical outlet controlled by a wall
switch because the device may be accidentally turned off.
Do not plug power cord into an outlet (or unplug it) with wet hands.
Do not submerge the device in liquids or pour cleaning liquids
over, into or onto the generator.
Do not use the generator if it is damaged, is not functioning
properly, or fails to meet an electrical safety check. Notify the
appropriate personnel to ensure the generator is removed from
service and properly repaired.
Failure on the part of all responsible individuals, hospitals,
or institutions, employing the use of Rez
ū
m Generator, to
implement the recommended maintenance schedule may
cause equipment failure and possible health hazards.
The manufacturer does not, in any manner, assume the
responsibility for performing the recommended maintenance
schedule. The sole responsibility rests with the individuals,
hospitals, or institutions utilizing the Rez
ū
m Generator.
If a critical error message is displayed, take the generator out
of service and call Boston Scientific Technical Services. Do not
attempt to service or maintain the generator while in use with
a patient.
If the generator measurement readings or messages seem dubious
or abnormal, check the condition of the patient first and stop using
the generator.
In the event of power failure, the generator will automatically shut
off. Turn the power button off. Please remove the Delivery Device
from the patient immediately per instructions in the Rez
ū
m Delivery
Device DFU. Turn on again to restart the generator to begin a new
therapy session.
No modification of this equipment is allowed. Do not attempt to
service or maintain the generator while in use with a patient.
RF Interference - Known RF sources, such as cell phones, radio or
TV stations, and two-way radios, may cause unexpected or adverse
operation of this generator. Consult qualified personnel regarding
system configuration.
Shock Hazard - Do not open, disassemble, or alter the Rez
ū
m
Generator. Failure to observe this warning can result in personal injury
or death. Refer maintenance issues to authorized service personnel.
The generator contains magnets in the LCD lid. Avoid close or
prolonged contact with electrical devices or devices that have
strong magnetic fields.
The generator is not intended to be deployed in settings or
situations that promote use by untrained personnel. Operation by
untrained personnel can result in injury or death.
The generator needs special precautions regarding
Electromagnetic Compatibility (EMC) and needs to be installed and
put into service according to the EMC information provided in this
operator’s manual.
The generator should not be used adjacent to or stacked with
other equipment. If adjacent or stacked use is necessary, test the
generator to verify normal operation. Refer to the Electromagnetic
Immunity information.
The Rez
ū
m Generator is equipped with a USB port that is sensitive
to ESD that may potentially result in injury or device failures.
The Rez
ū
m Generator is reusable but is restricted to a single patient
at a time for a therapy session.
To avoid the risk of electric shock, this equipment must only be
connected to a supply mains with protective earth.
Use a grounded AC outlet for the power supply and ground
this generator.
Use of accessories other than those specified in this document
may result in increased emission or decreased immunity of the
Rez
ū
m Generator.
Use only approved and specified parts, accessories, optional parts,
consumables, and components.
Use only specified power cord.
Use with the specified AC voltage and frequency.
When transporting the generator, it is important to position it with
the display facing away from the body.
PRECAUTIONS
None.
CAUTIONS
After cleaning, allow complete drying before plugging into an outlet.
Before conducting maintenance work, turn the power OFF and
unplug the power cord from the outlet to prevent electric shock.
Before moving this generator, turn the power OFF, remove
all accessories from the patient, and unplug the power cord from
the outlet.
Do not install this generator in the following locations:
• Locations where gases and flames are used.
• Locations where the air includes dust, salt, or sulfur.
• Locations exposed to prolonged direct sunlight.
• Locations that vibrate or are subject to sharp impacts.
• Locations near heating equipment.
• Locations where chemicals are stored.
• This generator cannot be used in any room in which noise-
generating apparatuses are used (such as an MRI room, CT
room, X-ray room, etc.).
Do not place anything on this generator.
Do not soak the generator or accessories in any medical liquid.
Also, keep liquids out of the generator and accessories.
Equipment operating in close proximity may emit strong
electromagnetic or radio frequency interference (RFI), which
could affect the performance of this device. Avoid operating
the Rez
ū
m Generator near cauterizers, diathermy equipment,
FM 2-way radios, or cellular phones. Turn power off to radio,
cellular and other like equipment near the Rez
ū
m Generator.
Refer to the EMI tables.
Exposing the Rez
ū
m Generator to extreme environmental
conditions outside of its specified parameters may compromise
the ability of the Rez
ū
m Generator to function properly and/or
cause the plastic to warp and/or crack.
Follow your facility’s procedures and applicable regulations
when disposing of anything that has been used on patients.
The generator should be at room temperature prior to use.
If there is condensation on the generator, dry it thoroughly
before turning the power on.
Input voltage range is 100 to 240V at 50 to 60 Hertz. Verify this
voltage matches that of the power outlet.
Observe the following cautions when connecting this generator
with other equipment:
• Ensure that the connected equipment is in accordance
with the IEC60601-1 or IEC safety standards.
• Employ additional protective measures (e.g., additional
protective earthing) as necessary.
Only approved equipment and accessories shall be connected
to the generator.
The Rez
ū
m Generator USB port is only intended for use by
authorized service personnel or to export treatment data.
The generator conforms to the requirements of the EMC
standards (IEC 60601-1-2:2007 and IEC 60601-1-2:2014), so it
can be used at the same time as other electrical simulators.
However, it may be affected by electrical scalpels and
microwave treatment devices and there may be an impact on
measurement precision for patients using cardiac pacemakers
and the like. Check the operation of this generator during and
after use of such equipment and with such patients.
The Rez
ū
m Generator is intended to be used indoors at a
medical facility or physician office environment only.
The Rez
ū
m Generator power cord and delivery device cable
may cause a trip hazard while attached to the generator.
The Rez
ū
m Generator needs special precautions regarding
Electromagnetic compatibility (EMC) and care should be taken
in accordance to the EMC information provided in this document.
To prevent damage to equipment, do not clean any part of the
generator with phenolic compounds. Do not use abrasive or
flammable cleaning agents. Do not steam, autoclave, or gas-
sterilize the generator.
Use of portable and mobile RF communications equipment near
the Rez
ū
m Generator may affect its operation.
Using this generator with the air vent blocked could cause a
breakdown. Clean this generator with care.
When any of the following occur, turn the power off, remove
all accessories from the patient, and unplug the power cord
from the outlet:
• There is smoke or a strange odor leaking out of the generator.
• The generator has been dropped or impacted by an object.
• Liquid or foreign matter gets inside the generator.
• If you think the generator may have been damaged.
When using disinfectant solutions, follow the
manufacturer’s directions.
Input voltage is pre-selected as labeled on the generator.
Please use caution in opening the shipping box and try not
to use sharp utility knives and such, as you risk cutting into
yourself and/or product.
ADVERSE EVENTS
The following adverse events have not been reported in the
clinical trials: erectile dysfunction, pelvic abscess, rectal wall
injury, and fistula. Delivering a form of thermal therapy or misuse
of the device has potential for producing these adverse effects.
Reported Adverse Events
The types of device related, or procedure related adverse
events reported are typical of thermal BPH ablation
procedures. There were no clinically significant complications
resulting from the treatment.
A summary of the adverse events observed in clinical studies is
presented in the Rez
ū
m Delivery Device Kit DFU.