ER900 and ER900 AF Event Monitors
13
Troubleshooting
Symptom Recommended solution
Install batteries or check battery direction.
Ensure patient electrodes/leads are connected to
patient properly.
Ensure patient cable is inserted completely.
Use only a one channel cable.
No single beep when inserting
patient cable
Lead loss is in off position, call technician.
Memory full-Phone Ring. Follow instructions To
Send and Erase Events.
Ensure patient cable is inserted completely.
Will not record
Ensure RECORD/SEND button is held for two
seconds.
Siren (alternating) tone while
recording
There is not a good connection. Check that
electrodes/leads have a good connection to patient
and cable is plugged into monitor
Phone ring sound every minute for
10 minutes
Memory is full, follow instructions To Send and
Erase Events.
Phone ring sound when
RECORD/SEND button is pushed
Memory is full, follow instructions To Send and
Erase Events.
Phone ring sound once An event is already stored in memory at start up,
also heard at the end of a recorded event. Follow
instructions To Send and Erase Events.
Three beeps every five minutes
with cable inserted
Batteries are low. Replace batteries and/or clean
battery contacts
Make sure mouthpiece of phone is directly over
monitor speaker
No information received by
receiving center
Ensure RECORD/SEND button is held for two
seconds.
Mouthpiece of phone must be close to the monitor
speaker hole.
Check telephone connection. Listen to phone line
before sending event(s) to ensure there is no noise.
Have patient call back and send ECG again.
Noise artifact on recorded ECG at
receiving center
Have patient try another phone.
Electrodes must be securely attached to patient.
Patient should remain still while recording.
Replace patient cable. Pulling on lead wires may
damage cable.
Verify the recording did not take place near a source
of electromagnetic interference (fluorescent lights,
computer monitors, or household appliances).
Noise artifact on recorded ECG at
patient location
Move electrodes slightly to the right or left of the
original location.
All or groups of timestamps for
recordings are the same.
If the inadvertent loss of power occurs, all the
timestamps in the FSK will reset to the time the unit
powered back up. Subsequent recordings will have
time stamps relative to the power up time.
Falling tone Transmission complete
Rising tone Ready to record
ER900 and ER900 AF Event Monitors
14
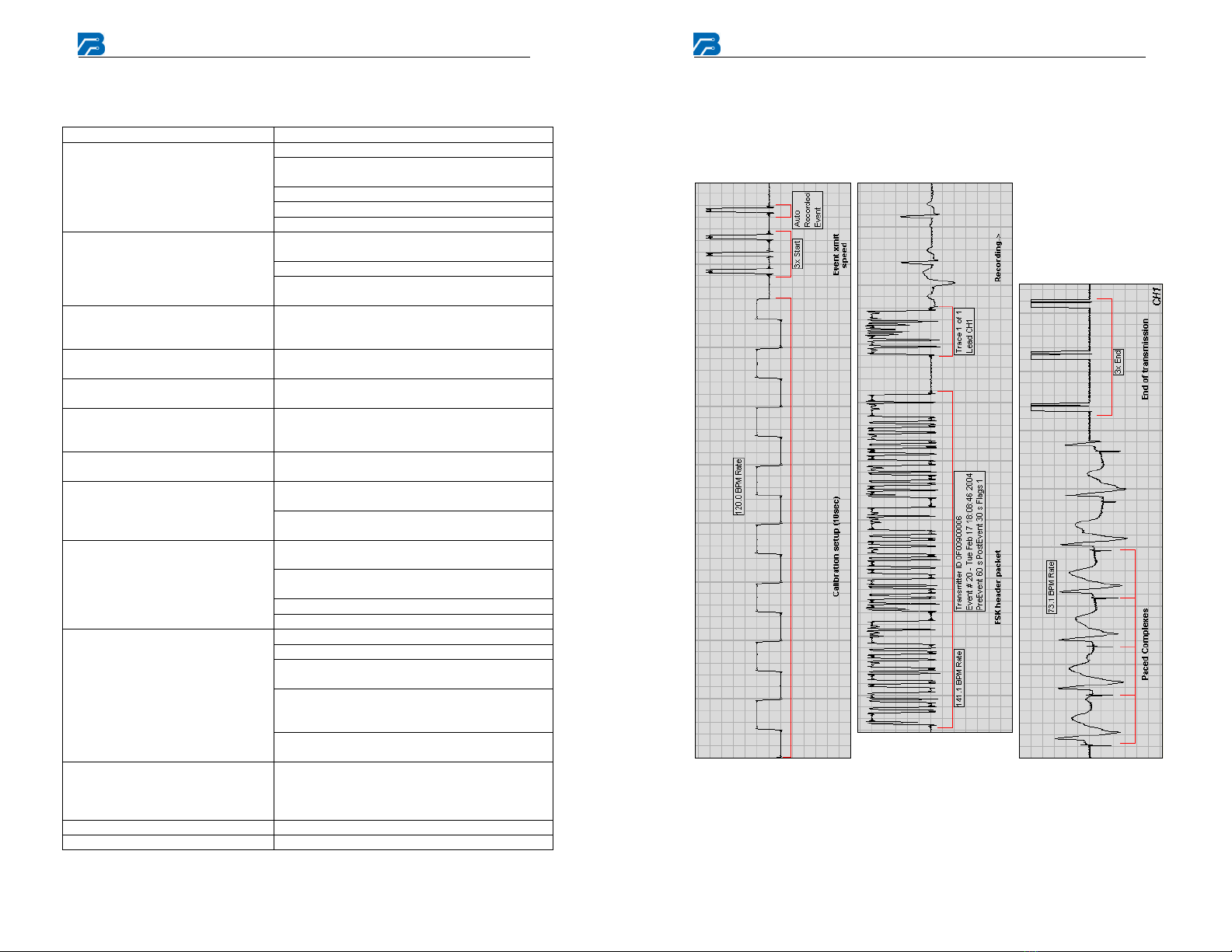
Event Markings
The following shows typical event markings that are generated by the
monitor and appear on the receiving station strip chart.