9
KOHJ-H-13-EN1-1.1-30112021
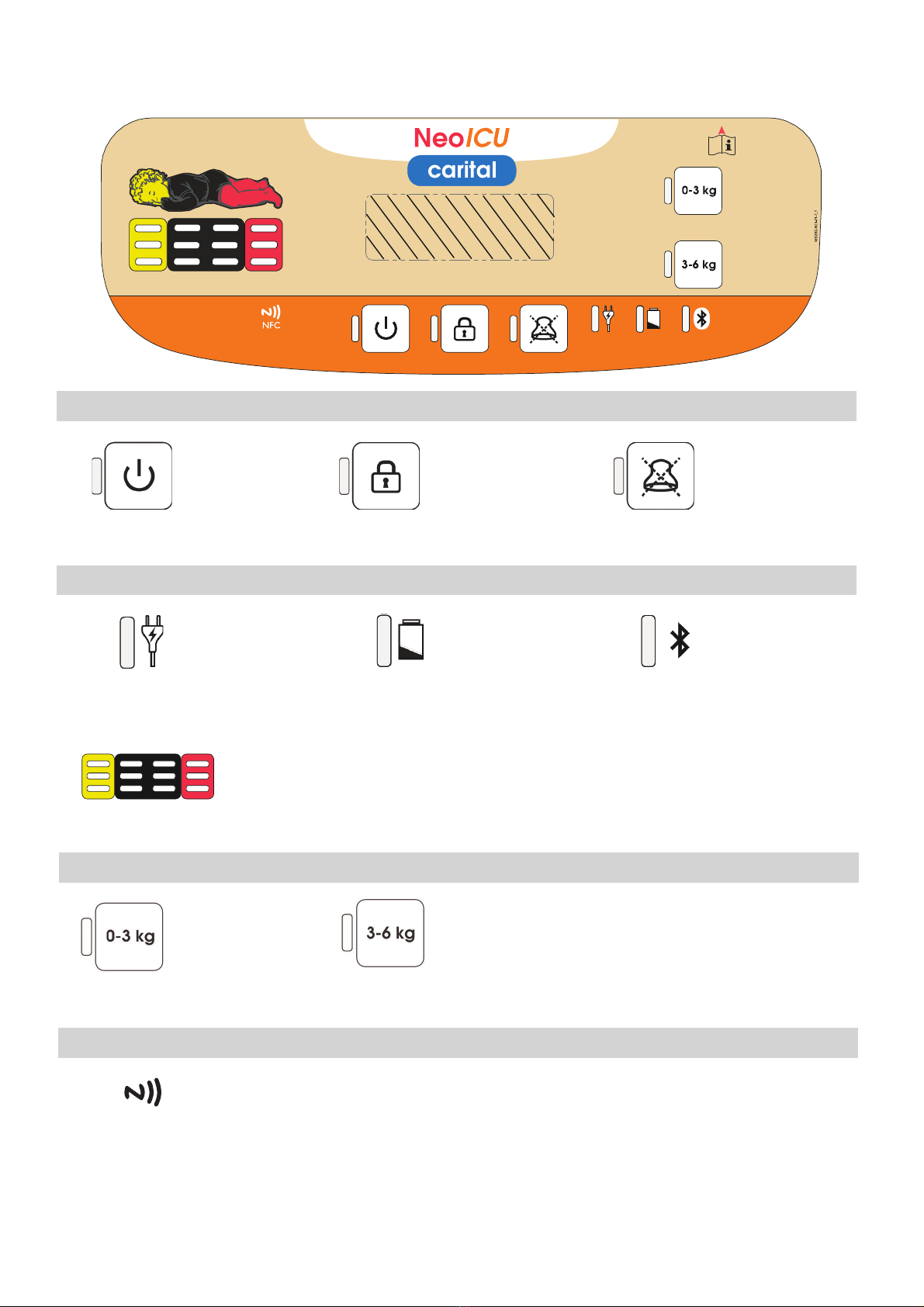
• Before placing the patient on the mattress, start the device as described in section
5.1, and allow the mattress system to adjust to the selected weight class successful-
ly, so that all green LEDs are lit in the centre of the LED light bar.
• The dimensions of the mattress should always fit to the size of the patient, such that
the pressure in all the adjustment sections are optimally placed to be regulated by
the controller in correspondence to the patient’s body parts.
• Before evacuation, disconnect the controller’s power cable from the mains.
• When resuscitating, turn offthe device from the standby button and start CPR im-
mediately without deflating the cells.
• Do not immerse the controller in liquid.
• Do not cover the controller while in operation.
• Be sure to put the quick guide caddy back in place after examination.
• Do not lift the mattress by holding the cells or the cover.
• Sharp objects may puncture the cells.
• If the cover or cells are exposed to urea (sweat and urine) for a prolonged period,
the molecular structure of polyurethane may break down, damaging the cover or the
cells. Clean the cover and/or cells immediately if exposed to urea.
• Do not clean the plastic parts of the mattress system using solvents, phenols or clean
alcohols.
• Ensure that the cover is entirely dry before commissioning it.
• Do not wash the foam inserts.
• If the mattress is used in violation of the instructions specified in the user’s guide,
or it is not cleaned of body secretions containing urea in particular, or the mattress
system is used by a prominently sweating or mobile patient, the estimated life cycle
of the cover and the cells may be shortened.
• Do not store anything on top of the mattress system.
• Do not place sharp or heavy objects on or near the mattress system.
• Keep the controller away from heat sources.
• Avoid using the controller in the proximity of other electric devices or in a stacked
configuration, as this may interfere with the controller’s operation. If the above use
is necessary, ensure the normal operation of the controller by monitoring it.
• Using accessories, transformers or cables other than those specified by the manufac-
turer or supplied with the device may result in elevated electromagnetic emissions
or reduced electromagnetic immunity and have adverse effect on the performance
of the controller for its intended purpose.