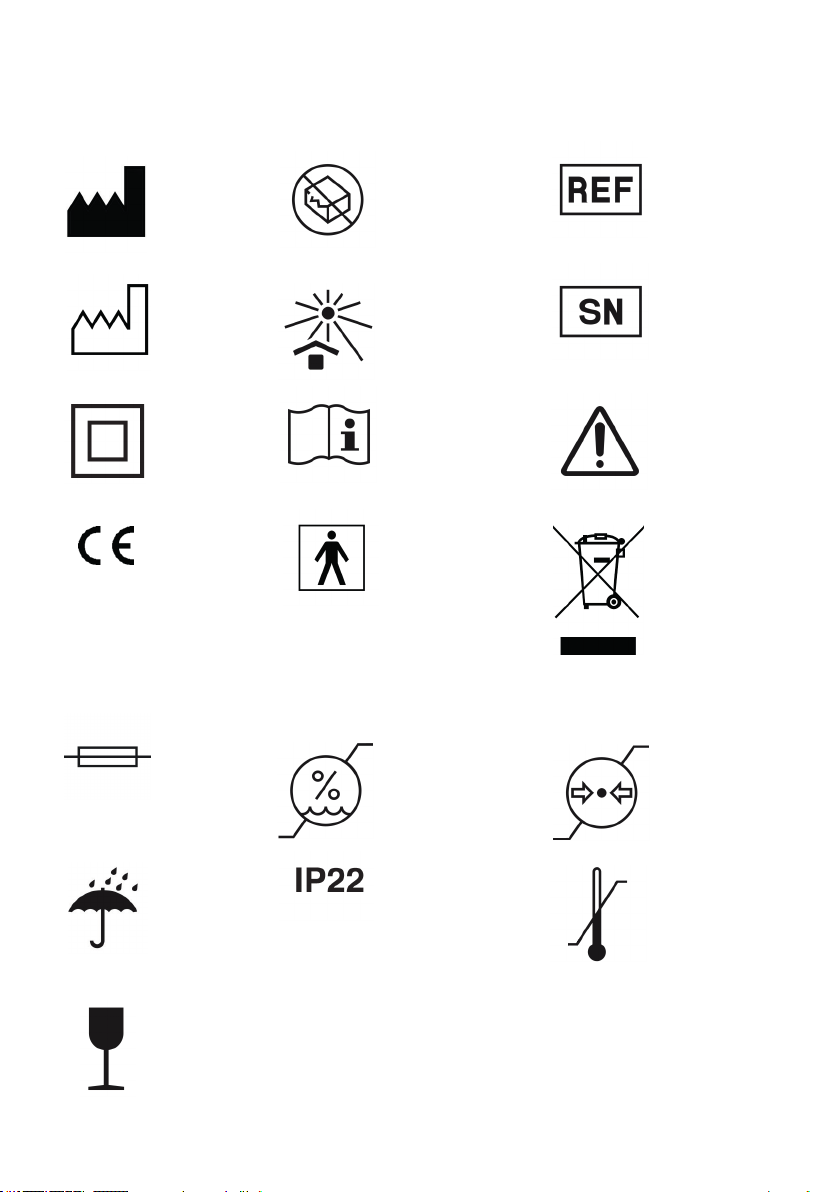
10
KOHJ-H-15-EN1-1.0-15102021
• Ensure that the cover is entirely dry before commissioning it.
• Do not wash the foam supports.
• Ifthemattressisusedinviolationoftheinstructionsspeciedintheuser’sguide,
or it is not cleaned of body secretions containing urea in particular, or the mattress
system is used by a prominently sweating or mobile patient, the estimated life cycle
of the cover and the cells may be shortened.
• Do not store anything on top of the mattress system.
• Do not place sharp or heavy objects on or near the mattress system.
• Keep the controller away from heat sources.
• Avoid using the controller in the proximity of other electric devices or in a stacked
conguration,asthismayinterferewiththecontroller’soperation.Iftheaboveuse
is necessary, ensure the normal operation of the controller by monitoring it.
• Usingaccessories,transformersorcablesotherthanthosespeciedbythemanufac-
turer or supplied with the device may result in elevated electromagnetic emissions
orreducedelectromagneticimmunityandhaveadverseeectontheperformance
of the controller for its intended purpose.
• The distance of portable devices communicating using radio frequencies (including
antenna cables and external antennas) to the controller and its cables should be at
least30cmsoastoensuretheperformancespeciedinthetechnicallesofthe
controller.
• The controller is intended for long-term use. However, it contains components that
may break if the product is dropped or subjected to impact or vibration exceeding
design standards. The limited manufacturer warranty does not apply to situations
where the product has been mishandled.
• The batteries may only be replaced by Carital®service. Incorrect battery replace-
ment may result in a situation where the device will not work correctly.
• Contaminated components must be cleaned before disposal or, if cleaning is not pos-
sible,disposedofinaccordancewithocialregulationspertainingtocontaminated
healthcare waste.
• If the controller has encountered a signicant mechanical strain (dropped, hard
collision or similar), check the mechanical condition of the control port's connection
gates and ensure that the seals between the operator panel/frame and the connec-
tion port/base plastic parts and the body are in place. If you notice any damage to
the device, contact Carital®service.
• Maintenance and repair must always be carried out by Carital®service. The user is
responsible for any and all consequences of the use of the device in a manner incon-
sistentwithitsintendedpurposeorresultingfrommaintenance,repairormodica-