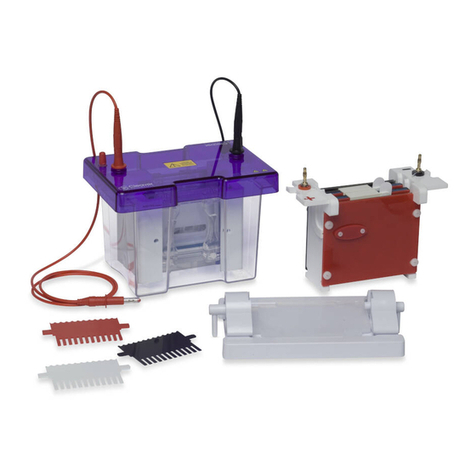
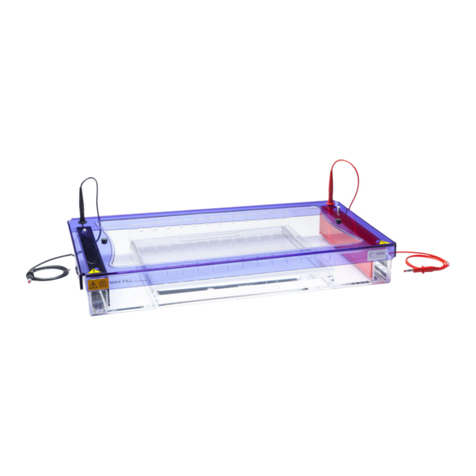
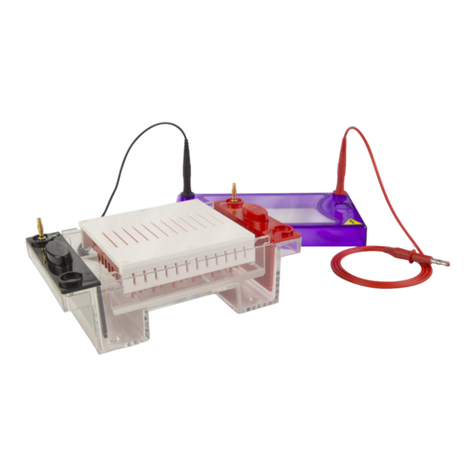
Cleaver Scientific multiSUB MSMINI7 User manual

multiSUB Horizontal
System


15/11/2018 Page 1
multiSUB®Horizontal
Electrophoresis Units
Instruction Manual
Catalogue Numbers
MSMINI7
MSMINI10
MSMINIDUO
MSMIDI7
MSMIDI10
MSMIDIDUO
MSCHOICE7
MSCHOICE10
MSCHOICE15
MSCHOICETRIO
MSCHOICEST20
MSCHOICEST25
MSMAXI10
MSMAXI15
MSMAXI20
MSMAXIDUO
MSMAXI25
Record the following for your records:
Model _____________________
Catalogue No. _____________________
Date of Delivery _____________________
Warranty Period _____________________
Serial No. _____________________
Invoice No. _____________________
Purchase Order No. _____________________

15/11/2018 Page 2
Contents
Instruction Manual 1
Catalogue Numbers 1
Contents 2
Safety Information 4
Packing List 5
Specifications 7
Operating Instructions 8
Usage Guidance and restrictions 8
Setting up the Horizontal Gel Tanks 8
Fitting Electrodes 8
Fitting Loading Guides 8
Gel Preparation 9
Gel Pouring 10
Casting Dams 10
Flexicaster 12
Tape 13
Running the Gel 13
Gel Staining and Viewing 14
Solutions 14
References 15
Troubleshooting 16
Care and Maintenance 18
Cleaning Horizontal Units 18
RNAse Decontamination 18
Ordering information 19
Comb options 21
Related Products 26
Warranty 28

15/11/2018 Page 3

15/11/2018 Page 4
Safety Information
When used correctly, these units pose no health risk. However, these units
can deliver dangerous levels of electricity and are to be operated only by
qualified personnel following the guidelines laid out in this instruction manual.
Anyone intending to use this equipment should read the complete manual
thoroughly. The unit must never be used without the safety lid correctly in
position. The unit should not be used if there is any sign of damage to the
external tank or lid.
These units comply with the following European directives:
2006/95/CE Low Voltage Directive and 2014/30/UE (official Title 2004/108/EC)
EMC Electromagnetic Compatibility
By virtue of the following harmonised standards:
BS EN IEC 61010-1: 2010 Safety Testing of Lab Equipment
BS EN IEC 61326-1:2013 EMC Electro Magnetic Compatibility

15/11/2018 Page 5
Packing List
Each multiSUB unit includes a tank, wired electrodes, lid and the following
items:
SKU Tray Tray Dams Combs Loading Guides Cables
MSMINI7 MS7-UV7, 7 x
7cm (W x L)
MS7-UVDAM,
Pack of 2
2 x MS7-8-1,
1mm, 8 sample
MS7-LG – Strips,
MS7-WP – Platform
CSL-
CAB
MSMINI10 MS7-UV10, 7
x 10cm (W x
L)
MS7-UVDAM,
Pack of 2
2 x MS7-8-1,
1mm, 8 sample
MS7-LG – Strips,
MS7-WP – Platform
CSL-
CAB
MSMINIDUO MS7-UV7,
MS7-UV10
MS7-UVDAM,
Pack of 2
2 x MS7-8-1,
1mm, 8 sample
MS7-LG – Strips,
MS7-WP – Platform
CSL-
CAB
MSMIDI7 MS10-UV7, 10
x 7cm (W x L)
MS10-UVDAM,
Pack of 2
2 x MS10-16-1,
1mm, 16
sample
MS10-LG – Strips,
MS10-WP –
Platform
CSL-
CAB
MSMIDI10 MS10-UV10,
10 x 10cm (W
x L)
MS10-UVDAM,
Pack of 2
2 x MS10-16-1,
1mm, 16
sample
MS10-LG – Strips,
MS10-WP –
Platform
CSL-
CAB
MSMIDIDUO MS10-UV7,
MS10-UV10
MS10-UVDAM,
Pack of 2
2 x MS10-16-1,
1mm, 16
sample
MS10-LG – Strips,
MS10-WP –
Platform
CSL-
CAB
MSCHOICE7 MS15-UV7, 15
x 7cm (W x L)
MS15-UVDAM,
Pack of 2
2 x MS15-20-1,
1mm, 20
sample
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB
MSCHOICE10 MS15-UV10,
15 x 10cm (W
x L)
MS15-UVDAM,
Pack of 2
2 x MS15-20-1,
1mm, 20
sample
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB
MSCHOICE15 MS15-UV15,
15 x 15cm (W
x L)
MS15-UVDAM,
Pack of 2
2 x MS15-20-1,
1mm, 20
sample
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB
MSCHOICETRIO MS15-UV7,
MS15-UV10,
MS15-UV15
MS15-UVDAM,
Pack of 2
2 x MS15-20-1,
1mm, 20
sample
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB
MSCHOICEST20 MS15-UVST20
15 x 20 cm,
(W x L)
MS15-UVDAM,
Pack of 2
4 x MS15-28MC-
1, 1mm thick, 28
sample MC
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB
MSCHOICEST25 MS15-UVST25
15 x 25 cm,
(W x L)
MS15-UVDAM,
Pack of 2
4 x MS15-28MC-
1, 1mm thick, 28
sample MC
MS15-LG – Strips,
MS15-WP –
Platform
CSL-
CAB

15/11/2018 Page 6
MSMAXI10 MS20-UV10,
20 x 10cm (W
x L)
MS20-UVDAM,
Pack of 2
2 x MS20-20-1,
1mm, 20
sample
MS20-LG – Strips,
MS20-WP –
Platform
CSL-
CAB
MSMAX15 MS20-UV15,
20 x 15cm (W
x L)
MS20-UVDAM,
Pack of 2
2 x MS20-20-1,
1mm, 20
sample
MS20-LG – Strips,
MS20-WP –
Platform
CSL-
CAB
MSMAXI20 MS20-UV20,
20 x 20cm (W
x L)
MS20-UVDAM,
Pack of 2
2 x MS20-20-1,
1mm, 20
sample
MS20-LG – Strips,
MS20-WP –
Platform
CSL-
CAB
MSMAXIDUO MS20-UV10,
MS20-UV20
MS20-UVDAM,
Pack of 2
2 x MS20-20-1,
1mm, 20
sample
MS20-LG – Strips,
MS20-WP –
Platform
CSL-
CAB
MSMAXI25 MS20-UV25,
20 x 25cm (W
x L)
MS20-UVDAM,
Pack of 2
2 x MS20-20-1,
1mm, 20
sample
MS20-LG – Strips,
MS20-WP –
Platform
CSL-
CAB
Packing List Checked by: ________________________
Date: ________________________
The packing lists should be referred to as soon as the units are received to
ensure that all components have been included. The unit should be checked
for damage when received.
Cleaver Scientific is liable for all missing or damaged parts / accessories within
7 days after customers have received this instrument package. Please contact
Cleaver Scientific immediately regarding this issue. If no response within such
period is received from the customer, Cleaver Scientific will no longer be liable
for replacement/damaged parts.
Please contact your supplier if there are any problems or missing items.

15/11/2018 Page 7
Specifications
1Power supply cables 1500V rated with retractable 4mm connectors
2 Safety lid and viewing pane High impact moulded acrylic construction
3 Height adjustable combs Acrylic
4 UV transparent gel tray UV transparent above 300nm, Moulded acrylic
5 Comb Slots Allow multiple combs per tray
6 Casting dams Natural rubber
7 Colour-coded electrodes Moulded acrylic, 99.99% platinum wire, gold plated
4mm plugs
8 Gel platform Notched to fix gel position
9 Safety lid thumb locators For safe removal of lid
10 Moulded buffer tank High Impact moulded acrylic construction for chemical
compatibility and shock resistance

15/11/2018 Page 8
Operating Instructions
Further information (including videos) regarding setting up and running the
multiSUB®units can be found at www.cleaverscientific.com
Usage Guidance and restrictions
Maximum altitude 2,000m.
Temperature range between 4°C and 65°C.
Maximum relative humidity 80% for temperatures up to 31OC
decreasing linearly to 50% relative humidity at 40OC.
Not for outdoor Use.
This apparatus is rated POLLUTION DEGREE 2 in accordance with IEC 664.
POLLUTION DEGREE 2, states that: “Normally only non-conductive pollution
occurs.
Occasionally, however, a temporary conductivity caused by condensation
must be expected”.
Setting up the Horizontal Gel Tanks
Fitting Electrodes
Note the position of the lid on the unit. This shows the correct polarity
and the correct orientation of the cables, black is negative and red
positive.
Remove the lid from the unit. Note if the lid is not removed, fitting the
cables may result in un-tightening of the gold plug and damage to the
electrode.
Screw the cables into the tapped holes as fully as possible so that there
is no gap between the lid and the leading edge of the cable fitting.
Refit the lid.
Fitting Loading Guides
These can be fitted to enhance visibility of the wells if desired. They can be
fitted to the white vinyl platform sheet or to the unit itself.
1. Seat the tray in the unit and note the position of the comb grooves. The
samples run black to red but the trays can be used frontward or
backwards so ensure that the comb grooves closest to the black
electrode are marked.

15/11/2018 Page 9
2. Remove the tray.
3. Peel the back off the loading guide and carefully apply the loading
guide directly to the gel platform.
The unit is now ready to be used.
Gel Preparation
The Table below shows the volume of agarose solution required to make the
desired agarose gel for each unit tray size. For a standard 0.7% agarose gel,
add 0.7 grams of agarose to 100 ml of 1x TAE or TBE solution. The same 1X
solution should be used in the tank buffer solution.
multiSUB® Mini Tray 7 x 7 cm 7 x 10 cm
Gel volume for a 5mm thick
gel
25 mL 35 mL
multiSUB® Midi Tray 10 x 7 cm 10 x 10 cm
Gel volume for a 5mm thick
gel
35 mL 50 mL
multiSUB®
Choice
Tray 15 x 7 cm 15 x 10 cm 15 x 15 cm
Gel volume for a 5mm thick
gel
52.5 mL 75 mL 112.5 mL
multiSUB®
Choice STRETCH
Tray 15 x 20 cm 15 x 25 cm
Gel volume for a 5mm thick
gel
150 mL 187.5 mL
multiSUB® Maxi Tray 20 x 10 cm 20 x 15 cm 20 x 20 cm
Gel volume for a 5mm thick
gel
100 mL 150 mL 200 mL
1. Add the agarose powder to a conical flask.
2. Add the appropriate amount of 1x TAE or TBE solution from the table
above. To prevent evaporation during the dissolving steps below, the
conical flask should be covered with parafilm.
3. Dissolve the agarose powder by heating the agarose either on a
magnetic hot plate with stirring bar or in a microwave oven. If using the
microwave method, the microwave should be set at around a 400 watt
or medium setting and the flask swirled every minute. The solution

15/11/2018 Page 10
should be heated until all crystals are dissolved. This is best viewed
against a light background. Crystals appear as translucent crystals.
These will interfere with sample migration if not completely dissolved.
The gel must be cooled to between 50°C and 60°C degrees before pouring.
Gel Pouring
The multiSUB®range of units allows three different methods of gel casting:
1. Casting Dams
2. Flexicaster
3. Traditional Tape
Casting Dams
1. To fit the casting dams, place one casting dam on the bench with the
groove facing upwards (1). Push the edge of the tray down firmly into
the groove (2). Repeat this for the other side (3). The dams should be
fitted so that there is no gap between the sides of the tray and the
groove in the dams. This will ensure that there is no possibility of gel
leakage.
2. Place the comb(s) in the grooves. Each tray has more than one comb
grove so that multiple combs can be used. Using multiple combs
increases sample number available per gel but decreases run length
and care must be taken to ensure that samples from the first wells do
not migrate into the lanes of the second comb wells.
3. Pour in the agarose carefully so as not to generate bubbles. Any
bubbles that do occur can be smoothed to the edge of the gel and
dispersed using a pipette tip.
4. Allow the agarose to set, ensuring that the gel remains undisturbed.

15/11/2018 Page 11
5. Carefully remove the gel casting gates and comb and transfer the gel
including tray to the main tank.
(1) (2) (3)

15/11/2018 Page 12
Flexicaster
1. Level the Flexicaster base by adjusting the feet so that the bubble is
exactly central.
2. Insert the desired length tray into the Flexicaster such that one end of
the tray is pushed up and seals against the silicone mat of the
permanent end of the Flexicaster.
3. Position the movable end of the Flexicaster so that the silicone mat is
pushed against the other end of the tray.
4. Turn the cam so that the silicone mat tightly seals against the side of the
tray. Pour in the agarose carefully so as not to generate bubbles. Any
bubbles that do occur can be smoothed to the edge of the gel and
dispersed using a pipette tip.
5. Allow the agarose to set, ensuring that the gel remains undisturbed.
6. Carefully remove the gel casting gates and comb and transfer the gel
including tray to the main tank.
MS7-FC and MS20-FC Flexicasters

15/11/2018 Page 13
Tape
1. Autoclave or plastic backed general tape should be used. A length
5cm longer than the width of each end of the tray should be cut. One
length should be placed over one end of the tray and stuck m1cm in
from the tray edge. This should then be folded, and the edges sealed
securely. Repeat for the other end and place onto a level surface for
gel pouring.
2. Place the comb(s) in the grooves. Each tray has more than one comb
grove so that multiple combs can be used. Using multiple combs
increases sample number available per gel but decreases run length
and care must be taken to ensure that samples from the first wells do
not migrate into the lanes of the second comb wells.
3. Pour in the agarose carefully so as not to generate bubbles. Any
bubbles that do occur can be smoothed to the edge of the gel and
dispersed using a pipette tip.
4. Allow the agarose to set, ensuring that the gel remains undisturbed.
5. Carefully remove the gel casting gates and comb and transfer the gel
including tray to the main tank.
Running the Gel
1. Mix the sample to be loaded with sample buffer – see solutions for
common sample buffers. Usually 3ul of sample buffer is adequate but
less may be used with sample volumes of less than 10ul.
2. Fill the unit with buffer until the gel is just flooded with buffer. This will give
the fastest resolution times. For enhanced quality of resolution of
sample, fill the unit to 5mm above the gel.
3. Load the samples into the wells using pipettes. Multi-channel pipettes
can be used for loading samples with MC compatible combs, see
listing in accessories for identification of these.
4. Carefully place the lid on the tank and connect to a power supply.
5. Typically, gels are run at between 90 and 150 volts. However, maximum
voltages are indicated on the serial badge of each unit. It should be
noted that higher voltages generally give faster but poorer quality
sample resolution.

15/11/2018 Page 14
Gel Staining and Viewing
The Multi Sub trays allow staining to be performed without removing the gel
from the tray if this is preferred.
1. Transfer the gel to a vessel containing the appropriate volume of 0.5 µg/ml
ethidium bromide stain for 15–30 minutes, see solutions for stock stain
concentration and adjust to the volume used accordingly. The entire gel
should be covered.
NOTE: Ethidium bromide is a suspected carcinogen and the necessary safety
precautions should be taken.
2. De-stain the gel for 10–30 minutes in distilled water again ensuring the gel
is completely immersed.
3. Rinse the gel twice for a couple of seconds with distilled water.
4. Transfer the gel to a UV Transilluminator.
The samples will often appear as brighter, clearer bands when
photographed or viewed using a gel documentation system. However, if the
gel bands are too faint then the staining procedure should be adjusted so
that there is less de-staining. If there is too much background, then the
staining procedure should be adjusted so that there is more de-staining.
Solutions
0.5M EDTA stock (500mL) dissolve in 400 ml distilled water:
93.05g EDTA disodium salt
Fill to 500 ml litre final volume with distilled water
50X TAE stock (1L) dissolve in 750 ml distilled water:
242 g tris base (FW = 121)
57.1 ml glacial acetic acid
100 ml 0.5 M EDTA (pH 8.0).
Fill to 1 litre final volume with distilled water

15/11/2018 Page 15
10X TBE stock (1L) dissolve in 750 ml distilled water:
108 g tris base (FW = 121)
55 g boric acid (FW = 61.8)
40 ml 0.5 M EDTA (pH 8.0)
Fill to 1 litre final volume with distilled water
Loading Dye
10x sample buffer stock consists of 50% glycerol, 0.25% bromophenol blue,
and 0.25% xylene cyanole FF in 1x TAE buffer. Only 1–10 ml of the 10x loading
dye should be prepared.
Ethidium Bromide Solution
Add 10 mg of Ethidium Bromide to 1 ml distilled water.
References
1. Sambrook, Fritsch, and Maniatis, Molecular Cloning A Laboratory
Manual, Second Edition, Cold Spring Harbor Laboratory Press, 1989.
2. Current Protocols in Molecular Biology, Greene Publishing Associates
and Wiley-Interscience, 1989.

15/11/2018 Page 16
Troubleshooting
Problem Cause Solution
Bands sharp but not enough
bands seen
Gel agarose percentage too
high
Incomplete digestion
Decrease agarose percentage.
Review enzyme activity, digest
further.
Band smearing and streaking
Agarose has improper
endosmosis
Salt concentration in sample too
high
Excessive power and heating
Sample spilled out of well
Incomplete digestion, nuclease
contamination, bad enzyme
Sample wells cast through the
gel. Sample leaks along bottom
of running surface
Reduce salt concentration to
≤0.1M.
Reduce voltage. See
electrophoresis instructions.
Apply sample carefully. Increase
gel thickness for large sample
volumes. Adjust comb height.
Heat sample. Review enzyme
activity. Digest sample further.
Comb should be placed to 1 to 2
mm above the base of the
running surface.
Curved line or distortion of bands Bubbles in sample wells Remove bubbles prior to
electrophoresis.
Curved bands, smiles Sample overload Reduce load.
Differential relative mobilities
Sample spilled out of wells
Unit not levelled
Samples should have proper
density. Apply carefully.
Level unit. Use a steady work
bench.
Gels crack
Too high voltage gradient,
especially with low melting
temperature agarose or low gel
strength gels
Reduce voltage. Run gel at lower
temperature.
High MW bands sharp; low MW
bands smeared Gel agarose percentage too low Increase agarose percentage.
Switch to polyacrylamide.
Ragged bands
Sample density incorrect
Sample well deformed
Excessive power or heating
See sample application
instructions.
Carefully remove comb,
especially from soft gels. Make
sure gel has solidified.
Cooling soft gels aids in comb
removal.
Reduce voltage. See
electrophoresis instructions.
Slanted lanes (bands) Gel not fully solidified
Comb warped or at an angle
Gel to solidify for at least 30-
45min.
Check alignment of comb.

15/11/2018 Page 17

15/11/2018 Page 18
Care and Maintenance
Cleaning Horizontal Units
Units are best cleaned using warm water and a mild detergent. Water at
temperatures above 60°C can cause damage to the unit and components.
The tank should be thoroughly rinsed with warm water or distilled water to
prevent build-up of salts, but care should be taken not to damage the
enclosed electrode and vigorous cleaning is not necessary or advised.
Air drying is preferably before use.
The units should only be cleaned with the following:
Warm water with a mild concentration of soap or other mild detergent.
Compatible detergents include dishwashing liquid, Hexane and Aliphatic
hydrocarbons
The units should not be left to in detergents for more than 30 minutes.
The units should never come into contact with the following cleaning agents,
these will cause irreversible and accumulative damage:
Acetone, Phenol, Chloroform, Carbon tetrachloride, Methanol, Ethanol,
Isopropyl alcohol, Alkalis.
RNAse Decontamination
This can be performed using the following protocol:
Clean the units with a mild detergent as described above.
Wash with 3% hydrogen peroxide (H2O2) for 10 minutes.
Rinsed with 0.1% DEPC-(diethyl pyro carbonate) treated distilled water,
Caution: DEPC is a suspected carcinogen. Always take the necessary
precautions when using.
RNaseZAP™(Ambion) can also be used. Please consult the instructions for
use with acrylic gel tanks.
This manual suits for next models
16
Table of contents
Other Cleaver Scientific Medical Equipment manuals
Popular Medical Equipment manuals by other brands

DARAY
DARAY MAG700 Range User manual & installation guide

Bien-Air Dental
Bien-Air Dental iChiropro quick start guide
Orliman
Orliman Manutec MF-52 Use and maintenance instructions

OsteoSys
OsteoSys SONOST 3000 user manual

Johnson & Johnson
Johnson & Johnson Sterilmed BluFlex DVT Instructions for use

Ferno
Ferno 24 miniMAXX user manual