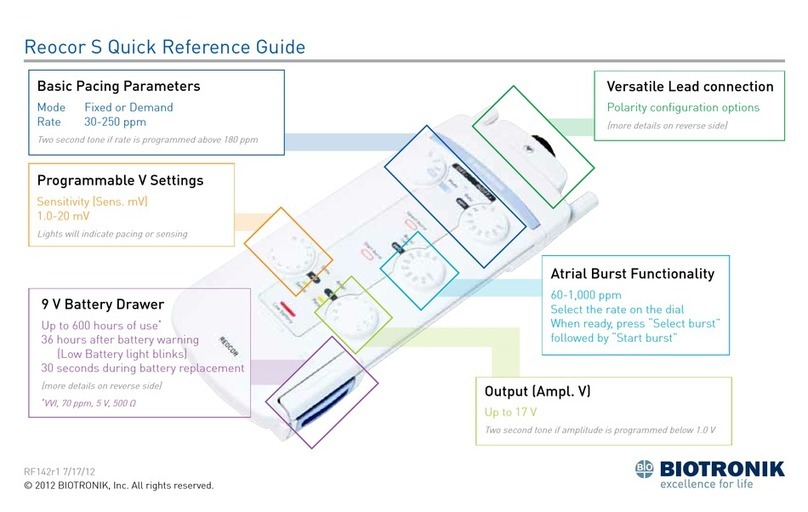
Chapter 1
BIOMONITOR IV Technical Manual
1
Chapter 1: Device Description
BIOMONITOR IV is a programmable, subcutaneous injectable cardiac monitor able to record
subcutaneous ECGs (sECGs) and other physiological parameters.
The BIOMONITOR IV is designed to automatically record the occurrence of arrhythmias in a
patient. Arrhythmia may be classified as atrial fibrillation (AF), bradyarrhythmia, pause, sudden
rate drop, or tachycardia. In addition, the BIOMONITOR IV can be activated by the patient to record
cardiac rhythm during symptomatic episodes.
Note - The BIOMONITOR IV subcutaneous ECG may differ from a surface ECG due to differences
in electrode separation and device placement in the body.
BIOMONITOR IV detects a subcutaneous ECG from a pair of electrodes. These signals are filtered
in two different ways. For detection of QRS complexes, the signals are filtered with a passband
of 10-40 Hz in order to suppress T-waves, artifacts, and baseline drift at low frequencies, and
myopotentials and EMI at high frequencies. The resulting signal is appropriate for QRS detection
as other components of the signal have been suppressed. This signal naturally does not have a
typical ECG morphology due to the bandpass. For waveform display (real-time streaming sECG
with the physician’s programmer and snapshots for review by the physician), a different passband
is utilized to retain signal features that may have diagnostic value. This passband is 0.5 – 40 Hz,
which is designed to retain morphological features of a typical ECG while still rejecting large low
frequency artifacts and baseline drift.
The BIOMONITOR IV system consists of three main components:
1. BIOMONITOR IV insertable cardiac monitor - The BIOMONITOR IV is a small, leadless
device that is typically inserted under the skin, in the chest. The device uses two
electrodes on the body of the device to continuously monitor the patient’s subcutaneous
ECG. BIOMONITOR IV can store up to 96 minutes (67 min minimum) of subcutaneous
ECG (sECG) recordings from both automatically detected arrhythmias and from
manually triggered symptom episodes. When a patient experiences symptoms, the
sECG recordings can be manually triggered by placing the Remote Assistant III over the
BIOMONITOR IV. The injectable cardiac monitor is provided preloaded in an insertion
tool. An incision tool is also provided.
2. BIOTRONIK Renamic / Renamic Neo Programmer – The programmer is used to set up
the BIOMONITOR IV to detect arrhythmias. It also allows one to view, save, or print the
stored information.
3. BIOTRONIK CardioMessenger®Smart is a telemetry patient device that forwards the
data from the BIOMONITOR IV to BIOTRONIK’s Home Monitoring Service Center.
BIOMONITOR IV may be used with BIOTRONIK Home Monitoring®technology, which is an
automatic, wireless, remote monitoring system for management of patients with insertable
cardiac monitors. When active, Home Monitoring enables the exchange of information about a
patient’s cardiac status from the implant to the Home Monitoring Service Center (HMSC) where
the physician may log in to view the data. The HMSC can be used to provide the physician with
advanced reports from the implanted device and process them into a graphical and tabular