Empi Hibresis 199589 User manual

Patch - Instructions For Use
Hybresis Patch Carton Contents:
• 6Patches
• 6SalineAmpules
• 6AlcoholPreps
REF: 199589
READ THE HYBRESIS CONTROLLER AND CHARGING STATION INSTRUCTIONS FOR USE FOR ADDITIONAL
IMPORTANT INFORMATION.
• Singleuseonly
• Non-sterile
• Disposable
• Storeatambienttemperature15°-30°C(59°-86°F)
• DONOTexposetotemperaturesabove50°C(122°F)
LATEX FREE
1
THEORY OF OPERATION
Iontophoresistransportschargedwater-solubledrugsandotherionicsubstancesacrossintact
skin.Iontophoresistechnologyisbasedontheprinciplethatanelectricpotentialcauses
chargedwater-solubledrugionsinsolutiontomigrateaccordingtotheirelectricalcharges.
Thedistributionofachargedionicdrugdeliveredbyiontophoresisisdependentuponthe
chargeoftheion,thesizeoftheion(molecularweight),thestrengthanddurationofthe
electricalcurrentapplied,thecompositionofthePatchandnumerousotherfactors.
DESCRIPTION
TheHybresisSystemdeliverschargedwater-solubledrugsandotherionicsubstancesacross
intactskinandconsistsofthreecomponents:aChargingStation,rechargeableController(s)
anddisposablePatch(es).
TheHybresisSystemisdesignedtoprovidethefollowingthreetreatmentoptions:
Hybresis Treatment
TheControllerdeliverscurrentat3mAtothePatchforthreeminutesforaSkinConductivity
Enhancement(SCE),followedbythepatientwearingthePatchforapproximatelyonetotwo
hours,resultingina40-80mA-minutetreatmentrespectively.
Standard Treatment
TheControllerdeliverscurrentat2,3or4mAtothePatchfor10-20minutes,resultingina40
mA-minutetreatment.Foran80mA-minutetreatment,repeatthetreatment.
Patch-Only Treatment
ThePatchdeliverslowlevelcurrentover2-4hours,resultinginanapproximate40-80
mA-minutetreatmentrespectively.
Warnings
• Keepoutofthereachofchildren.
• Donotapplyelectrodessuchthatthecurrentpathwaycrossestheheartorbrain,assafety
hasnotbeenestablished.
• Advisethepatienttoremoveelectrodesifanyunduesensationofpainorburningoccurs
duringthetreatmentandtoreportdiscomforttoclinic.
• Toestablishgoodcontactbetweentheelectrodesandskin,excessivehairmaybeclipped,
butDONOTSHAVE.Shavingmaycauseskinbreaksthatarenotreadilyseenandcanincrease
theriskofadverseskinreactions.
• Smallpinheadsizeblistersmayresultinresponsetocertaindrugs.Contactphysicianif
problempersistslongerthan24hours.
• Onrareoccasions,iontophoresistherapycanresultintransientskinreactionssuchasrash,
inflammationandirritation.Theseskinreactionsmaybetheresultofindividualsensitivityto
theionicsolutionused,theconditionoftheskinattheonsetoftreatment,reactiontothe
materialsintheelectrodes,orapoorconnectionbetweentheelectrodeandthepatient’s
skin.Advisethepatientofthispossibilitybeforestartingtreatment.Ifavisibleskinreaction
doesoccur,instructthepatienttodiscontinuethetreatmentandconsulttheprescribing
physician.
• Caremustbetakenwhenoperatingthisequipmentadjacenttoorstackedwithother
equipment.Potentialelectromagneticorotherinterferencecouldoccurtothisortotheother
equipment.Careshouldbetakentominimizethisinterferencebynotusingotherequipment
inconjunctionwithit.
• Thesystemisnotsuitableforuseinthepresenceofaflammableanaestheticmixturewithairor
withoxygenornitrousoxide.
3
INDICATIONS
TheHybresisSystemisindicatedfortheadministrationofsolublesaltsorotherdrugsintothe
bodyformedicalpurposesasanalternativetohypodermicinjection.
CONTRAINDICATIONS
·Cardiacpacemakers-Donotuseonpatientswithpacemakersorotherimplanteddevices.
·Drugsensitivity–Donotuseonpatientswithknownsensitivitytothedrugbeing
administered.
·Pregnancy–Donotuseonpregnantwomen.Thesafetyofthesystemusedduringpregnancy
hasnotbeenestablished.
·Scarring–Donotuseondamagedskin,denudedskinorotherrecentscartissue.
·Skinsensitivity–Donotuseonpatientswithknownsensitivitytoelectricalcurrentortothe
solutionbeingadministered.
·Headtreatment–Donottreatacrosseitherthetemporalregionortheorbitalregion.
2
STANDARD MODE TREATMENT
NOTE:WhileusingtheController,shouldanin-processiontophoresistreatmentneedtobe
stoppedorpaused,DONOTsuddenlyremovetheControllerfromthePatchwithoutrst
switchingotheController.TostoporpauseatreatmentwhiletheControllerisadministering
iontophoresis,presstheON/OFFbuttonandwaitafewmomentsfortheControllertoturno.
1.PresstheON/OFFbuttonontheController.TheGreenHybresisLightwillblinkslowly.
2.PushtheStandardModebuttonontheController.The2mAindicatorlightwillblinkslowly.
Eachadditionaldepressionofthebuttonwillscrolltothenextsetting-3mAor4mA.
3.AttachtheControllertothePatch.ThePatchconnector(locatedatthecenterofthePatch)
plugsintotheslotonthebackoftheController.EnsurethatthePatchconnectorisfullyand
securelyengagedintotheController-aclickwillbehearduponfullengagement.
4.PositionthepatientsothatthereisnopressureonthePatchduringtreatment.
5.PresstheSTARTbuttontobegintreatment.TheGreenmALightwillblinkmorerapidly,
thenglowsteadily.
6.TochangetheiontophoresiscurrentsettingwhiletheControllerisadministeringa
treatment,presstheStandardModebuttontore-selectthedesiredsetting.Withinafew
moments,theControllerwillautomaticallyadjusttothenewsetting.
7.In10-20minutes(see following table)theControllerwillsoundabeepandthelightswill
turno,indicatingthe40mA-minutetreatmenthasbeencompleted.
StandardMode 40mA-min 80mA-min
2mA 20minutes Repeatsteps1-2and5-7
3mA 13minutes Repeatsteps1-2and5-7
4mA 10minutes Repeatsteps1-2and5-7
8.RemovetheControllerfromthePatch.
9.RemoveanddiscardthePatchafterthetreatmenthasbeencompleted.ThePatchcannot
bereused. 9
Patch-Only Treatment
ThePatchdeliverslowlevelcurrentover2-4hours,resultinginanapproximate40-80
mA-minutetreatmentrespectively.
HYBRESIS MODE TREATMENT
NOTE:WhileusingtheController,shouldanin-processiontophoresistreatmentneedto
bestoppedorpaused,DONOTsuddenlyremovetheControllerfromthePatchwithout
rstswitchingotheController.TostopatreatmentwhiletheControllerisadministering
iontophoresis,presstheON/OFFbuttonandwaitafewmomentsfortheControllertoturno.
1.PushtheONbuttonontheController.TheGreenHybresisLightwillblinkslowly.
2.AttachtheControllertothePatch.ThePatchconnector(locatedatthecenterofthePatch)
plugsintotheslotonthebackoftheController.EnsurethatthePatchconnectorisfullyand
securelyengagedintotheController-aclickwillbehearduponfullengagement.
3.PositionthepatientsothatthereisnopressureonthePatchduringtreatment.
4.PresstheSTARTbuttontobegintreatment.ThegreenHybresisLightwillblinkmorerapidly,
thenglowsteadily.
5.Afterthreeminutes,theControllerwillsoundabeepandthelightswillturno
automatically.ThisindicatesthattheSkinConductivityEnhancement(SCE)iscompleted.
6.RemovetheControllerfromthePatch.ThePatchwillnowcontinuetodelivertheremainder
oftheiontophoresistreatmenttothepatient.
7.Theaveragetimetocompletethedoseisindicatedinthefollowingtable.Toprevent
excessivedosing,thePatchautomaticallyswitchesoiontophoresisafterthemaximum
dosehasbeenadministered.
HybresisMode 40mA-minutes 60mA-minutes 80mA-minutes
WearTime 1hour 1.5hours 2hours
8.InstructthepatienttoremoveanddiscardthePatchafteraminimumofonetotwohours
fora40to80mA-minutedoserespectively.
9.DiscardthePatchaftertreatmenthasbeencompleted.ThePatchcannotbereused.
8
EmpiInc.
599CardiganRoad
St.Paul,MN55126-4099USA
651.415.9000
800.328.2536
360352Rev.C,©2008Empi9/08
ManufacturedintheUSA
ThisproductmaybeprotectedbyoneormoreofthefollowingU.S.patentsownedorlicensedbyEmpi:5,087,242;5,413,628;5,573,563;6,761,977;
6,653,014.02aswellasforeignequivalents.Otherpatentspending.
PATCH-ONLY TREATMENT
1.Theaveragetimetocompleteapatch-onlydoseisindicatedinthefollowingtable.
Patch 40mA-minutes 60mA-minutes 80mA-minutes
WearTime 2hours 3hours 4hours
2.InstructthepatienttowearthePatchpertheappropriatedoseandtimeindicatedinthe
tableasaminimum.Topreventexcessivedosing,thePatchautomaticallyswitcheso
iontophoresisafteramaximumdosehasbeenadministered.
3.RemoveanddiscardthePatch.ThePatchcannotbereused.
10
Precautions
• Consultdirectionsfortheuseofthedrug/compoundbeforedelivery.Somedrugs/
compoundsrequireaspecificpolarizationforuse.Observetheindications,contraindications,
warningsandprecautionsrelatedtothisissue.
• Donotuseelectrodesthathavebeenpreviouslyused.
• Inspecttheelectrodesbeforeuse.Discardanyelectrodethatshowssignsofalterationor
damage,astheseelectrodesmaynotbesafeforuse.
• Theelectrodescanbewornduringnormalactivity.However,excessivemotionwherethe
electrodeshavebeenplacedcancausepoorcontactbetweentheskinandtheelectrodeor
unevendistributionofcurrent,resultingingreaterriskofskinirritation.
• Atransienterythematousreaction,characterizedbyauniformredpattern,cansometimes
occurdirectlyundertheelectrodes.Therednessusuallydisappearswithinafewhoursafter
treatment.Advisethepatientofthispossibilitybeforestartingtreatment.
• Useonlysalineampulesuppliedforthereturnpad.Forpositivepolarity(+),useonlydrugs
withChloride(Cl-)counterions.Useoftapwateroranyothersolutionmaycausetattooingor
staining.
• Handlethesystemwithcare.Donotimmersethesysteminfluidsorallowittobeconnected
withotherelectricaldevices.Donotdrop,abuse,orinanywayexceednormaluse.Donot
sterilize.
• Donotoperatethissysteminanenvironmentwhereotherdevicesarebeingusedthat
intentionallyradiateelectromagneticenergyinanunshieldedmanner.Portableandmobile
RFcommunicationsequipmentcanaffectMedicalElectricalEquipment.
4
a company
DJOglobal.com
360352B Hybresis Map.indd 1 10/29/08 12:37:54 PM
205 Hwy 22 East
Clear Lake, SD 57226
651.415.9000
800.328.2536
360352 Rev. E © 2008, 2015 Empi, Inc.
Warnings
• Keep out of the reach of children.
• Do not apply electrodes such that the current pathway crosses the heart or brain, as safety
has not been established.
• Advise the patient to remove electrodes if any undue sensation of pain or burning occurs
during the treatment and to report discomfort to clinic.
• To establish good contact between the electrodes and skin, excessive hair may be clipped,
but DO NOT SHAVE. Shaving may cause skin breaks that are not readily seen and can increase
the risk of adverse skin reactions. Do not apply over broken or compromised skin (e.g.,
sunburns, cuts, or acne) due to increased risk of skin reaction.
• Small pinhead size blisters may result in response to certain drugs. Contact physician if
problem persists longer than 24 hours.
• On rare occasions, iontophoresis therapy can result in transient skin reactions such as rash,
inflammation and irritation. These skin reactions may be the result of individual sensitivity to
the ionic solution used, the condition of the skin at the onset of treatment, reaction to the
materials in the electrodes, or a poor connection between the electrode and the patient’s
skin. Advise the patient of this possibility before starting treatment. If a visible skin reaction
does occur, instruct the patient to discontinue the treatment and consult the prescribing
physician.
• Care must be taken when operating this equipment adjacent to or stacked with other
equipment. Potential electromagnetic or other interference could occur to this or to the other
equipment. Care should be taken to minimize this interference by not using other equipment
in conjunction with it.
• The system is not suitable for use in the presence of a flammable anaesthetic mixture with air
or with oxygen or nitrous oxide.
• Do not wear electrode or controller during Magnetic Resonance Imaging (MRI) scans as this
may result in metal overheating and causing skin burns in the area of the patch.
Manufactured in the USA

PATCH DESCRIPTION
TheHybresisPatchisadisposable,single-usePatchwithaninternalbatteryandcurrent-
limitingcircuitry.Itcandeliverbothnegativelyandpositivelychargedwater-solubledrugs/
compounds.ThePatchcanbeusedwithandwithouttheController.TheControllerisused
withthePatchtodeliveraHybresisorStandardTreatment.APatch-OnlyTreatmentdoesnot
requiretheuseoftheController.
Drug Polarity Labeling -
ThepolarityofthedrugpadsarelabeledonthePatch.
Patch Drug Pads -
ThePatchhastwodrugpads.Eachdrugpadhasa~1.5mLllvolume.Usenegativelycharged
water-solubledrugs/compoundsonthenegative(-)drugpadandpositivelychargedwater-
solubledrugs/compoundsonthepositive(+)drugpad.
Battery Pack and Controller Connector -
TheBatteryPackthatcontainstwo3-Voltbatteriesisalsotheconnectinglocationforthe
Controller.
PREPARING THE PATIENT
Advisethepatientthationtophoresishasthepotentialtoresultinskinirritationand/orburns.
·Directcurrentmayresultintransienterythemaunderthepads.Theerythemagenerallyresolves
itselfwithinafewhours.
·Usecautionwhentreatingpatientswithsensitiveskinorthosewhomayhavedicultyhealing.
1.Advisethepatientthationtophoresiscausesmildtingling,pricklingand/orawarm
sensation.Thisisnormalandshouldbeanticipatedbythepatient.
5
2.Advisethepatienttoreportimmediatelyanypainduringtreatment.Ifthepatientcomplains
ofpain,pausethetreatment,inspecttheareaunderthePatchandmakeanynecessary
adjustments(e.g.repositionthePatchtoensurefullskincontact,decreasecurrent,etc.)
beforeresumingthetreatment,ordiscontinuethetreatment.
3.AdvisethepatienttoremoveanyjewelrythatmaycomeincontactwiththePatch.
PREPARING THE PATCH
1.TearopenthesealedtreatmentkitandremovethePatch.
2.PlacethePatchonaatsurfacewiththeabsorbentpadsfacingup.
3.Cleanthetreatmentsitethoroughlywithalcoholprepbyrubbingforsixtoeightsecondsto
removedryskin,oilsandothercontaminants.Allowthetreatmentsitetodrycompletely.
CAUTION: Failuretocleanskinthoroughlymaycauseexcessiveskinirritationorburns.
NOTE: ThePatchwillnotadheresucientlytoskinwithlotion,oilordirt.
NOTE: Cliphairifnecessarytoimproveskincontact.DONOTshave.
4.Place~1.5mLofawater-solubledrug/solutiononappropriatepolaritypad(activepad).On
theotherpad(returnpad),apply~1.5mLofsuppliedsalineampule.Usenegativelycharged
water-solubledrugsonthenegative(-)drugpadandpositivelychargedwater-soluble
drugsonthepositive(+)drugpadtoactivelydeliverthedrug.For(+)polarity,useonly
drugswithChloride(Cl-)counterions.
NOTE:Fillvolumeisapproximately~1.5mL.Drugpadsshouldbesaturatedbutnot
overlled.Ifthedrugpadsareoverlledbeyondthesaturationpoint,thepadswillleakand
directlyaecttheadhesionofthepatchtothetreatmentsite.
CAUTION: Failuretoevenlydistributedrugorsalineontoactiveorreturnpadsmay
causeexcessiveskinirritationorburns.
DONOTllPatchwhileitisonthepatient.
DONOToverorunderlldrugpads.
6
Service
Forclinicalquestions,contacttheProfessionalServicesDepartmentat1-800-328-2536
ext.8506.IfanycomponentoftheHybresisSystemisnotfunctioningproperlyorrequires
servicing,contacttheEmpiRepairDepartmentat1-800-862-2343foraReturnGoods
Authorization(RGA).
Whenreturninganyproducts,pleaseincludeyourname,address,phonenumberanda
descriptionoftheproblem.
Returnto:
Empi
Attn:RepairDepartment
47492SDHwy.22
ClearLake,SD57226USA
Ordering
Order Number Description
199586 HybresisChargingStation
199587 HybresisController
199589 Hybresis Patch
700566 ChemoblockVialVentingSystem
11
Guidance and manufacturer’s declaration – electromagnetic immunity
The Hybresis Patch is intended for use in the electromagnetic environment specified below. The customer or
the user of the Hybresis Patch should assure that it is used in such an environment.
Immunity test IEC 60601
test level
Compliance level Electromagnetic
environment - guidance
Electrostatic discharge
(ESD)
IEC 61000-4-2
±6kV contact
±8kV air
±6kV contact
±8kV air
Floors should be wood,
concrete or ceramic tile. If
floors are covered with
synthetic material, the
relative humidity should
be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2kV for power supply
lines
±1kV for input/output
lines
±2kV for power supply
lines
±1kV for input/output
lines
Mains power quality
should be that of a typical
commercial or hospital
environment.
Surge
IEC 61000-4-5
±1kV differential mode
±2kV common mode
±1kV differential mode
±2kV common mode
Mains power quality
should be that of a typical
commercial or hospital
environment.
Voltage dips, short
interruptions and voltage
variations on power
supply input lines
IEC 61000-4-11
<5% UT
(>95% dip in UT) for 0,5
cycle
40% UT
(60% dip in UT) for 5
cycles
70% UT
(30% dip in UT) for 25
cycles
<5% UT
(>95% dip in UT) for 5 sec
<5% UT
(>95% dip in UT) for 0,5
cycle
40% UT
(60% dip in UT) for 5
cycles
70% UT
(30% dip in UT) for 25
cycles
<5% UT
(>95% dip in UT) for 5 sec
Mains power quality
should be that of a typical
commercial or hospital
environment. If the user
of the Hybresis Patch
requires continued
operation during power
mains interruptions, it is
recommended that the
Hybresis Patch be
powered from an
uninterrupted power
supply or a battery.
Power frequency
(50/60Hz) magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency
magnetic fields should be
at levels characteristic of
a typical location in a
typical commercial or
hospital environment.
NOTE UTis the a.c mains voltage prior to application of the test level.
13
Guidance and manufacturer’s declaration – electromagnetic emissions
The Hybresis Patch is intended for use in the electromagnetic environment specified below. The customer or
the user of the Hybresis Patch should assure that it is used in such an environment.
Emission tests Compliance Electromagnetic environment -
guidance
RF emissions
CISPR 11
Group 1 The Hybresis Patch uses RF energy
only for its internal function.
Therefore, its RF emissions are very
low and are not likely to cause any
interference in nearby electronic
equipment
RF emissions
CISPR 11
Class A The Hybresis Patch is suitable for
use in all establishments, including
domestic establishments and
those directly connected to the
public low-voltage power supply
network that supplies buildings
used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations
IEC 61000-3-3
Complies
12
Recommended separation distances between portable and mobile RF communications equipment
and the Hybresis Patch
The Hybresis Patch is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the Hybresis Patch can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and the Hybresis Patch as recommended below, according to the maximum output power of
the communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d= [3,5]√P
V1
80 MHz to 800 MHz
d= [3,5]√P
E1
800 MHz to 2,5 GHz
d= [7]√P
E1
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance
din meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P
is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
15
Guidance and manufacturer’s declaration – electromagnetic immunity
The Hybresis Patch is intended for use in the electromagnetic environment specified below. The customer or
the user of the Hybresis Patch should assure that it is used in such an environment.
Immunity test IEC 60601
test level
Compliance level Electromagnetic environment -
guidance
Portable and mobile RF
communications equipment
should be used no closer to any
part of the Hybresis Patch,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.
Recommended separation
distance
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
3 V d= [3,5]√P
V1
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2,5 GHz
3 V/m d= [3,5]√P80 MHz to 800 MHz
E1
d= [7]√P800 MHz to 2,5 GHz
E1
where Pis the maximum output
power rating of the transmitter in
watts (W) according to the
transmitter manufacturer and dis
the recommended separation
distance in metres (m).
Field strengths from fixed RF
transmitters, as determined by
an electromagnetic site surveya,
should be less than the
compliance level in each
frequency rangeb.
Interference may occur in the
vicinity of equipment marked
with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
aField strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and
land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted
theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in the location in which the
Hybresis Patch is used exceeds the applicable RF compliance level above, the Hybresis Patch should be
observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as reorienting or relocating the Hybresis Patch.
bOver the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.
14
GLOSSARY OF SYMBOLS
Thisdevicemaycontainoneorallofthefollowingsymbols:
RefertoInstructionManual/Booklet
CouncilDirective2002/96/ECconcerningWasteElectricalandElectronicEquipment
(WEEE).IndicatesarequirementnottodisposeofWEEEasmunicipalwaste.Contact
yourlocaldistributorforinformationregardingdisposaloftheunitandaccessories.
TypeBFEquipment
UnderwritersLaboratoriesInc.,indicatesproductmeetsUSandCanadianproduct
safetystandards.ThisdevicecomplieswithUL60601-1andCSAC22.2
No.601-1-M90.
PrecautionaryInstructions
SingleUseOnly
“On”/“Off”(Push-Push)
ForPrescriptionOnly
DONOTusedrugsthatarenotwater-soluble.
DONOTusedrugsuspensions.
DONOTuseaPatchthatappearsalteredordamaged.
DONOTapplyPatchtodirty,oilyorlotionedskin.
Useoftapwaterornon-chloridedrugsolutiononpositivepolaritymaycausetattooing
orstaining.
5.Makesurethatthetreatmentsitehasintactskin.
CAUTION: Failuretofollowtheseguidelinesmayresultinskinirritationorburns.
WARNING: DONOTapplythePatchoverdamagedordenudedskinorotherrecentscar
tissue,skinwithingrownhair,pimples,razornicksorskinwithwoundsthathavenothealed.
6.RemovetheadhesivereleaselinerfromthehydratedPatch.
7.ApplythehydratedPatchsothatthedrugpadisoverthetreatmentsiteandsecureitby
pressingtheadhesiveborder.Avoidpressingdirectlyoverthepads.Pressingdirectlyonthe
padscancauseleakagethatwillcompromiseadhesiontothepatient.
NOTE: DONOTtapeorbindthePatchduringtreatment.Donotapplyhotorcoldtherapyover
Patchduringtreatment.
ADMINISTERING TREATMENTS
TheHybresisSystemisdesignedtoprovidethefollowingthreetreatmentoptions:
Hybresis Treatment
TheControllerdeliverscurrentat3mAtothePatchforthreeminutesforaSkinConductivity
Enhancement(SCE),followedbythepatientwearingthePatchforapproximatelyonetotwo
hours,resultingina40-80mA-minutetreatmentrespectively.
Standard Treatment
TheControllerdeliverscurrentat2,3or4mAtothePatchfor10-20minutes,resultingina40
mA-minutetreatment.Repeatthetreatmentforan80mA-minutedelivery.
7
360352B Hybresis Map.indd 2 10/29/08 12:37:55 PM
EXPECTED LIFE AND DISPOSAL
• The Best If Used By date for the Patch is shown on the Patch package. The patches may not be
effective if they are used past the Best If Used By Date.
• DO NOT REUSE patches that have been previously used as these patches have been designed
for single use only. Reuse may cause burns to the patient. After the treatment discard the
used patch out of the reach of children and pets and according to local, state and federal
regulations for Lithium Manganese Dioxide button cell batteries. Do not dispose of the Patch
in fire or an incinerator.
SERVICE
For clinical questions, contact the Professional Services Department at 1-800-328-2536
ext. 8506. If any component of the Hybresis System is not functioning properly or requires
servicing, contact the Empi Repair Department at 1-800-862-2343 for a Return Goods
Authorization (RGA).
When returning any products, please include your name, address, phone number and a
description of the problem.
Return to: Empi, Inc.
Attn: Repair Department
47492 SD Hwy. 22,
Clear Lake, SD 57226 USA
ORDERING
Order Number Description
199586 Hybresis Charging Station
199587 Hybresis Controller
199589 Hybresis Patch
700566 Chemoblock Vial Venting System
GLOSSARY OF SYMBOLS
This device may contain one or all of the following symbols:
Refer to Instruction Manual/Booklet
Council Directive 2002/96/EC concerning Waste Electrical and Electronic Equipment
(WEEE). Indicates a requirement not to dispose of WEEE as municipal waste. Contact
your local distributor for information regarding disposal of the unit and accessories
Type BF Equipment
Underwriters Laboratories Inc., indicates product meets US and Canadian product
safety standards. This device complies with UL 60601-1 and CSA C22.2
No. 601-1-M90.
Precautionary Instructions
Single Use Only
“On” / “Off” (Push-Push)
For Prescription Only
Do not wear electrode or controller during Magnetic Resonance Imaging (MRI) scans
as this may result in metal overheating and causing skin burns in the area of the
patch.
11N1
DO NOT use drugs that are not water-soluble.
DO NOT use drug suspensions.
DO NOT use a Patch that appears altered or damaged.
DO NOT apply Patch to dirty, oily or lotioned skin.
Use of tap water or non-chloride drug solution on positive polarity may cause tattooing
or staining.
5. Make sure that the treatment site has intact skin.
CAUTION: Failure to follow these guidelines may result in skin irritation or burns.
WARNING: DO NOT apply the Patch over damaged or denuded skin or other recent scar
tissue, skin with ingrown hair, pimples, razor nicks or skin with wounds that have not healed
and sunburned skin.
6. Remove the adhesive release liner from the hydrated Patch.
7. Apply the hydrated Patch so that the drug pad is over the treatment site and secure it by
pressing the adhesive border. Avoid pressing directly over the pads. Pressing directly on the
pads can cause leakage that will compromise adhesion to the patient.
NOTE: DO NOT tape or bind the Patch during treatment. Do not apply hot or cold therapy over
Patch during treatment.
ADMINISTERING TREATMENTS
The Hybresis System is designed to provide the following three treatment options:
Hybresis Treatment
The Controller delivers current at 3 mA to the Patch for three minutes for a Skin Conductivity
Enhancement (SCE), followed by the patient wearing the Patch for approximately one to two
hours, resulting in a 40-80 mA-minute treatment respectively.
Standard Treatment
The Controller delivers current at 2, 3 or 4 mA to the Patch for 10-20 minutes, resulting in a 40
mA-minute treatment. Repeat the treatment for an 80 mA-minute delivery.
meters
Popular Controllers manuals by other brands

Rain Bird
Rain Bird ESP-LXD Installation, programming & operation guide

Alcor Micro
Alcor Micro AU9368 Technical reference manual
Halma
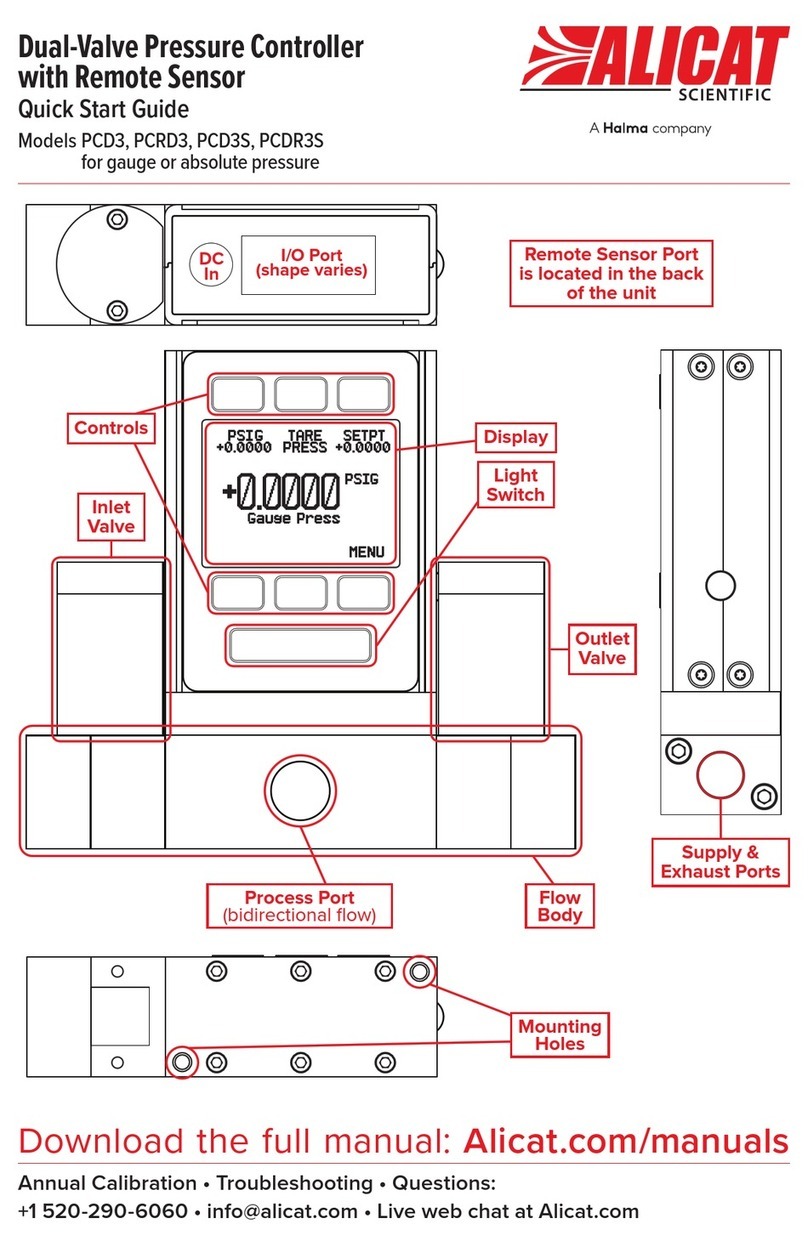
Halma ALICAT SCIENTIFIC PCD3 quick start guide

Comunello
Comunello MOWIN FAST 50/160 instruction manual

Fife
Fife CDP-01 Additional instruction manual

Milltronics
Milltronics EnviroRanger ERS500 instruction manual