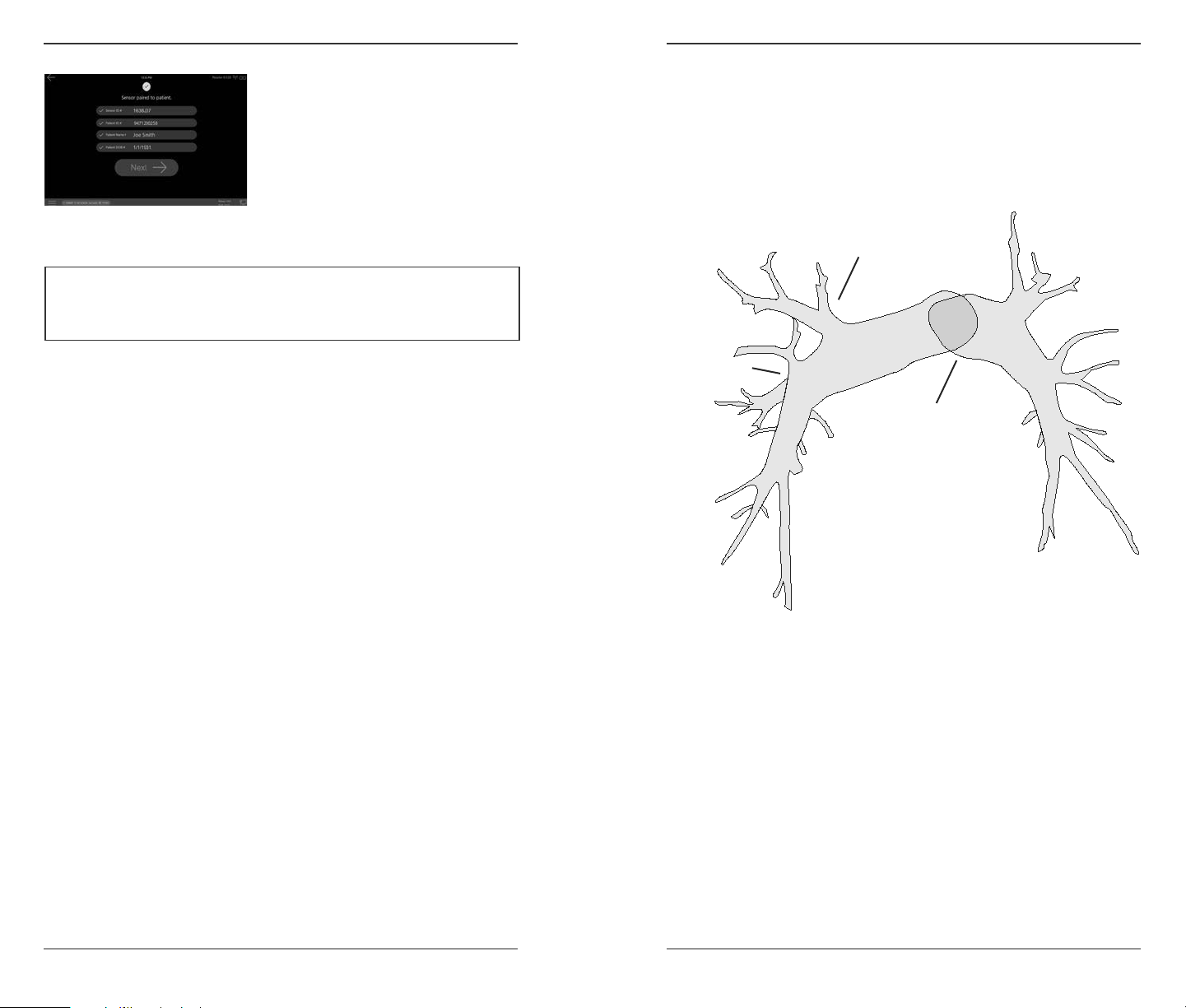
Instructions For Use | 1514 | © 2018 Endotronix Inc. 100474-00 Rev 1
• myCordella Patient Reader may be interfered with by other equipment
generating electromagnetic fields. When possible, avoid using the Reader
while simultaneously using other equipment within ~1.5 meters such as:
patient monitoring systems, chest EKG leads, motors on motorized beds,
pagers, laptop computers, tablets, cell phones, cordless phones, wireless
routers, air conditioners, or walkie talkies (450MHz devices) within ~3
meters.
• The Reader requires special precautions regarding electromagnetic
compatibility (EMC) and needs to be placed into service according to
the EMC information provided. If interference is noted (e.g. if CalEQ and
Reader continue to disconnect), remove or stop using the interfering
equipment.
• Portable RF communications equipment (including peripherals such as
antenna cables and external antennas) should be used no closer than
~1.5 meters to any part of the Reader. Otherwise, degradation of the
performance of the Reader could result.
• The patient should not have necklaces, jewellery, shirt pocket contents,
metal objects, and near field communication objects in the vicinity of the
reading location while taking a reading.
• Under certain conditions, the Reader’s surface may exceed 41°C. If the
Reader becomes too warm to hold comfortably, place it back in the
Docking Station and wait for several hours for it to cool. If the Reader
remains too warm to hold comfortably for more than a day, contact
Customer Support.
• If the skin becomes red, warm, or irritated, immediately stop using the
Reader and contact Customer Support.
• DO NOT use more than one Reader in the same general vicinity at one
time, as use of multiple Readers at once may cause them to interfere
with each other.
3.3 Precautions
• The Delivery System should only be used with a guidewire. Do not
aspirate or infuse the Delivery System guidewire lumen during use.
• The implant procedure is an adjunct to a standard (RHC) procedure. All
standard protocols for the RHC should be followed.
• The Cordella Sensor is attached to the distal end of the Delivery System;
care should be taken to limit contact with the Sensor prior to insertion
through the introducer sheath.
• Ensure all Luer connections are secure (but not over-tightened) prior to
insertion through the introducer. If at any time during the procedure the
Stability Sheath comes loose from the Torque Catheter, reconnect and
tighten the Luer connection to secure until after Sensor deployment.
• Removing the Cordella Sensor after implantation is not recommended.
• If there is evidence of a change in device performance, please contact
Customer Support.
• The Cordella Sensor and Delivery System are only compatible with a 14
French introducer or larger. Use of a smaller introducer may damage
the Cordella Sensor or Delivery System product and may prevent
introduction. Endotronix does not recommend use of peel away
introducers.
• Precaution should be taken to avoid disruption of the Cordella Sensor
position after deployment with the Delivery System or any subsequent
devices used during right heart catheterization procedures.
• Torqueing the Stability Sheath with the Torque Catheter removed may
result in kinking of the Stability Sheath and may impact the reliability of
a fluid-filled pressure measurement.
• Precaution should be taken to avoid damage to the Cordella Sensor prior
to implantation. It is an all-glass enclosure and it is fragile. Only remove
from packaging when ready to start a procedure. Take care to avoid
shock or drop to the distal end of the Delivery System where the Cordella
Sensor is pre-mounted. Care should be taken to limit contact with the
Cordella Sensor prior to insertion through the introducer sheath.
• Avoid squeezing or pinching the body of the Cordella Sensor if at all
possible.
Warnings: Reader and Docking Station (Cont.)