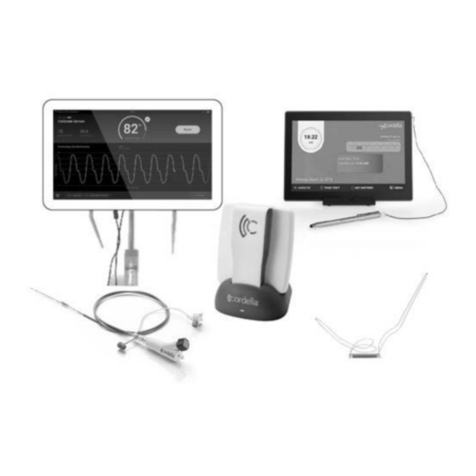
Endotronix my cordella User manual

© 2019 Endotronix® Inc. 100469-00 Rev. 2
Patient Reader Manual
IMPORTANT
PLEASE READ THIS MANUAL BEFORE USING THE
CORDELLA™PULMONARY ARTERY SENSOR SYSTEM
CAUTION--Investigational device. Limited by Federal
(or United States) law to investigational use.
815 Ogden Ave
Lisle, IL 60532
www.endotronix.com
1-888-512-5595
Endotronix Ireland Limited
DCU Alpha Innovation Centre
Old Finglas Road, Glasnevin
Dublin 11, D11 KXN4
Ireland

2
© 2019 Endotronix® Inc. 100469-00 Rev. 2
(this page left blank intentionally)

3
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Table of Contents
Introduction...................................................................................... 4
The Cordella™ Pulmonary Artery Sensor System............................... 5
Safety Information ............................................................................ 6
Patient Privacy and Medical Privacy Standards ................................. 7
Implant procedure ............................................................................ 7
Before the Implant Procedure........................................................... 7
The Implant Procedure...................................................................... 8
After the Implant Procedure ............................................................. 9
Getting Started................................................................................ 10
Reader Placement ........................................................................... 10
Taking a Reading ............................................................................. 11
Audio & Visual Cues ........................................................................ 13
Reader Audio Cues .......................................................................... 13
Reader Visual Cues.......................................................................... 13
Docking Station Visual Cues ............................................................ 14
Traveling with the myCordella Patient Reader and Docking Station 14
Contact Us....................................................................................... 15
Repairing Equipment....................................................................... 15
Return Materials Authorization (RMA)............................................ 16
Inspection & Cleaning Instructions.................................................. 16
MRI Conditions................................................................................ 17
APPENDICES.................................................................................... 18
Appendix A: Cordella™Pulmonary Artery Sensor System
Components .................................................................................... 18
Appendix B: Equipment Specifications ............................................ 19
Appendix C: Electromagnetic Guidance .......................................... 21
Definition of Symbols...................................................................... 26

4
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Introduction
The Cordella™ Pulmonary Artery (PA) Sensor System is intended to
connect healthcare professionals and patients with tools designed to
improve comprehensive heart failure management. This guide
provides basic information about instructions for use of the Cordella™
Pulmonary Artery Sensor System in the home.
NOTE: The information provided in this patient manual does not
attempt to define any intervention, health care policy, or procedures.
Clinical procedures and policies are the responsibility of your doctor.
Managing Heart Failure with Daily Cordella™ Sensor Readings
The Cordella Sensor is designed to provide your care team with
information about the pressure in your PA in order to view and act on
changes in PA pressure or trends over time. The wireless Cordella
Sensor is permanently implanted in a vessel between the heart and
lungs. When you take a measurement, the Cordella Sensor sends a
signal which indicates the pressure in the artery. Changes in PA
pressure may indicate fluid accumulation in the lungs.
By watching the trends in your PA pressure, your healthcare providers
may intervene to address the increasing pressure before you have
symptoms. This information, along with your other vital signs such as
blood pressure, heart rate, blood oxygen, weight and responses to
health-related questions provides your care team with a snapshot of
your health status on the secure website, myCordella Patient
Management Portal (PMP). Based on the trends, your healthcare
provider can manage your heart failure proactively and remotely,
potentially improving your quality of life and preventing
hospitalizations.
Intended Use
The Cordella PA Sensor System is intended to measure, record, and
transmit PA pressure data from NYHA Class III heart failure patients at
home to clinicians for assessment and patient-centered heart failure
management.

5
© 2019 Endotronix® Inc. 100469-00 Rev. 2
The Cordella™ Pulmonary Artery Sensor System
Cordella Pulmonary Artery Sensor System is an innovative
myCordella™ peripheral designed for on-demand measurement of PA
pressure from your home. This may help your healthcare providers
identify pulmonary congestion suggestive of worsening heart failure
through trends in PA pressures.
Component Name
myCordella™ Patient Reader
(Reader)
Device that collects pulmonary artery pressure
data from the Cordella Sensor. Each day, you will
hold the wireless Reader against your chest for
approximately 18 seconds, guided by audio and
visual cues on the myCordella™ Hub and Reader.
The Reader sends data to the Hub provides daily
PA pressure readings to your clinician on the
myCordella™ Patient Management Portal,
enabling a more complete picture of your health
status.
myCordella™ Docking Station
(Docking Station)
Small stand that conveniently holds and charges
the Patient Reader while you are not using it. The
Reader should always remain in the Docking
Station unless it is in use or it is packed for
traveling. LED lights on the front will indicate if the
Reader is charging or fully charged.
Cordella™ Pulmonary Artery Sensor
(Cordella Sensor)
Small implant that resides permanently in the
pulmonary artery. The Cordella Sensor will enable
PA pressure measurements with the myCordella
Patient Reader while at home. The Reader will
collect and securely transmit PA pressure readings
to the clinician. Measurements from this device
are equivalent to those obtained by trained clinical
personnel using invasive, fluid-filled catheter-
based products.

6
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Safety Information
To prevent personal injury or damage to any equipment, please read
and observe all safety information.
Warnings
•The Reader is suitable for home healthcare environments and professional healthcare
facilities except for near active HF surgical equipment and the RF shielded room of an ME
system for magnetic resonance imaging, where the intensity of EM disturbance is high.
•The Reader and Docking Station should not be used adjacent to or stacked with other
equipment. If it is necessary to operate the components adjacent to or stacked with other
equipment, verify that the system is operating normally in the configuration in which it will be
used. If necessary, contact customer service to help re-locate the system.
•DO NOT expose any power accessories to water or other liquids.
•DO NOT disassemble or modify any component of the Cordella™ PA Sensor System.
•DO NOT use myCordella™ in the presence of explosive or flammable anesthetic agents.
•The Cordella™ PA Sensor System is not intended for emergency use or real-time monitoring.
•The Cordella™ PA Sensor System is not intended to be an emergency response device. In case
of a medical emergency, call the local Emergency Medical Services and/or your healthcare
provider.
•After the implantation procedure, it is critical to adhere to prescribed anticoagulation and
other medications from the physician.
•Power cables may pose a tripping hazard. Be mindful of cords crossing walkways.
•myCordella™ Patient Reader may be interfered with by other equipment generating
electromagnetic fields. When possible, avoid using the system while simultaneously using the
equipment within ~5 feet/1.5 meters such as: laptop computers, tablets, e-readers, cell
phones, cordless phones, wireless routers, hair dryers, electric shavers, refrigerators, home
stereos, alarm-clock radios, air conditioners, electric ovens, washers, dryers, dishwashers,
televisions, microwaves, or walkie talkies (450MHz devices) within ~10 feet/3 meters.
•The Reader requires special precautions regarding electromagnetic compatibility (EMC) and
needs to be placed into service according to the EMC information provided. If interference is
noted, remove or stop using the interfering equipment.
•Use only the cables and accessories provided. The use of accessories, transducers or cables
other than those specified or provided as replacement parts, may result in decreased
immunity of the system, inaccurate readings, damage to the system, injury to user, or
improper operation.
•Portable RF communications equipment (including peripherals such as antenna cables and
external antennas) should be used no closer than ~5 feet/1.5 meters to any part of the
Reader. Otherwise, degradation of the performance of the Reader could result.
•Under certain conditions, the Reader's surface may exceed 41°C. If the Reader becomes too
warm to hold comfortably, place it back in the Docking Station and wait for several hours for it
to cool. If the Reader remains too warm to hold comfortably for more than a day, contact
customer service.
Warnings
This symbol indicates “the possibility of system
damage or malfunction, delay in receipt of
information to a doctor, inaccurate readings, or
injury.”
Precautions
This symbol indicates “the possibility of system
damage, malfunction, or the delay in treatment.”

7
© 2019 Endotronix® Inc. 100469-00 Rev. 2
•If the skin becomes red, warm, or irritated, immediately stop using the Reader and contact
customer service.
•Contact Endotronix customer service if more than 1 Cordella user resides in the same home.
DO NOT use more than one Reader in the same general vicinity at one time, as use of multiple
Readers at once may cause them to interfere with each other.
•The Reader contains Lithium Ion batteries. DO NOT place the Reader on a hot surface.
Precautions
•Avoid exposing any components of myCordella™ to water or liquids. Contact customer service
for a replacement if any components are exposed to liquids.
•DO NOT drop the Reader. Handle with care.
•If dropped, the Reader may expose the battery. If the battery is exposed, contact Endotronix®
immediately for a replacement Reader. Any damage to the Reader may result in an inaccurate
reading.
•DO NOT use the Reader if the plastic casing has been damaged, cracked or any component
becomes dislodged.
•If the Reader label becomes compromised, contact Endotronix customer service.
•Accuracy of the Cordella™ PA Sensor System is affected by a change in body temperature (<-
3mmHg/∆°C).
•Accuracy of the Cordella™ PA Sensor System is slightly affected by large changes in elevation
between the initial baseline calibration and subsequent measurements. Readings may lose
accuracy when taken >2000m of elevation.
•The Cordella Sensor is a permanent implant. Removing the implant after implantation is not
recommended.
•The Cordella Sensor may be affected by a change in elevation above or below sea level. If you
plan to travel below sea level or SCUBA dive or extremely high altitudes without
pressurization, please contact customer service.
Patient Privacy and Medical Privacy Standards
All components of the Cordella PA Sensor System have been designed
to comply with patient privacy regulations. Your healthcare providers
organization as well as Endotronix® will ensure that they are also in
complete compliance with privacy and security statutes, as well as the
organization’s policies, procedures, and protocols to respect patient
information and ensure patient privacy at all times.
Endotronix® is not responsible for the use or abuse of patient
information by health care providers.
Implant procedure
Before the Implant Procedure
Prior to the implant procedure, you and your doctor will discuss the
benefits and risks of the procedure and the Cordella PA Sensor

8
© 2019 Endotronix® Inc. 100469-00 Rev. 2
System. You will be given a detailed description of the procedure, your
doctor will discuss the risks associated with the procedure and the
Sensor, your doctor will respond to any questions you have, and you
will be asked to sign an informed consent.
Inform your doctor of any infections, arrhythmias, or bleeding that
occurs before the implant procedure.
The Implant Procedure
During a right heart catheterization, the Cordella Sensor is
permanently implanted in a blood vessel that carries blood from the
heart to the lungs. This catheterization is a standard procedure used
to measure the pressures in the heart and PA.
The Cordella Sensor is the length of a $0.01 coin (19.3mm x 3.8mm x
1.9mm) and has two wire loops extending off either end which hold it
in place in the vessel. The Cordella Sensor is tied down on a catheter
that is skillfully navigated to your PA by your doctor.
The steps of the implant procedure are:
1. You may be mildly sedated to start but your doctor will likely
need you awake during certain parts of the procedure to
follow instructions.
2. A nurse will clean the access site and will numb it with a local
anesthetic.
3. You will be monitored by an electrocardiogram with sensors
on different areas of your body to measure electrical activity
of your heart. These patches will be attached to wires to
monitor your heart during the procedure.
4. Once the anesthetic has numbed the access site, your doctor
will make a small incision and insert a small tube called a
pulmonary catheter. The pulmonary catheter will be
threaded through your vein while your doctor looks at a
screen with a live x-ray—called a fluoroscope—of your veins
and heart. Your doctor will navigate this catheter through
your veins to your heart and then to your PA.
5. Your doctor will take a few pictures with the fluoroscope at
this point by injecting dye through the end of the catheter
that is still outside your body. These pictures will give your

9
© 2019 Endotronix® Inc. 100469-00 Rev. 2
doctor a better idea of what the vessels in your pulmonary
arteries look like and will help guide placement of the sensor
to the best possible position. This is called angiography.
6. At this point the pulmonary catheter is removed, the
catheter with attached Cordella Sensor replaces it, and the
best possible position for the Sensor is confirmed.
7. Once the Sensor is in the best possible position, your doctor
will implant it.
8. After the Cordella Sensor is implanted, the handheld Reader
will be held over the right side of your chest and the Reader
will begin reading the PA pressure. A pulmonary catheter will
be used to take some simultaneous reference pressure
measurements to calibrate the new Sensor.
9. Following the removal of all catheters, the access site will be
closed, leaving the Cordella Sensor in the PA.
After the Implant Procedure
Once the procedure is complete, you will be brought to a recovery
area. While in the recovery area, a staff person will come by to take
some PA pressure measurements with the Reader. You may be kept
overnight for observation.
You will be trained on proper use of the Reader and the optimal
(prescribed) location for the Reader for readings taken at home. You
will also be given an implant card (about the size of a business card) and
discharged to go home. The implant card must always be kept with you
to alert medical and security personnel to important information about
your Sensor.

10
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Getting Started
The myCordella™ Patient Reader will automatically pair with the
myCordella Hub on start-up. No additional user input is required.
Upon return home with the myCordella Reader and Docking Station,
connect the Docking Station Power Cord to the power port on the
back of the Docking Station and the other end into the wall outlet.
Place the Docking Station on a flat surface near the myCordella Hub
with a minimum two-inch/five-centimeter clearance from
surrounding objects or walls.
Place the Reader in the Docking Station. The LED light on the front of
the Docking Station will blink while charging and change to solid white
when the Reader is charged. Call Endotronix customer service if this
does not occur within 24 hours of setup.
The Reader is distributed in Travel Mode. Start the Reader for the first
time by holding the travel button on the bottom for several seconds.
Reader Placement
Reliably and repeatedly finding proper Reader location is essential to
collecting valid PA pressure measurements.
Position the Reader on the prescribed
location (from training), most likely
the upper right chest. A clinician may
indicate that the Reader should be
placed in a different location—for
example on the side of the body. The
Reader will wait for good signal
strength before gathering information
from the Sensor, so adjust the position
until the Reader plays the “Sensor
Located” tone and displays a quickly
flashing blue light (see the Audio &
Visual Cues section below).

11
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Taking a Reading
The handheld Reader should be removed from the Docking Station
using the left hand for ease of locating the Cordella™ Sensor in the
right PA (right side of the chest). It is essential for proper operation of
the Reader to maintain a metal free zone in the area where the
reading will take place and to take each reading in a consistent body
position.
Remove all necklaces, watches and jewelry prior to
beginning the measurement within 3 inches/8 cm of your
prescribed reading location.
Allow the Reader to charge fully before obtaining your first
home reading.
Ensure that you are sitting upright
comfortably and resting for a few minutes
until breathing comfortably. To see the LED
indicator on the Reader, use a mirror or ask a
caregiver to describe it as you take the
reading. The animation walks you through
proper data collection using the Reader.
Briefly:
1. Check to ensure the LED light on the
front of the Docking Station indicates a
battery charge that will allow for the
reading to be completed. The LED on the
Docking Station should be solid white
when the Reader is fully charged and
ready to take a reading.
2. Remove the Reader from the Docking
Station with the left hand to make it
easier to locate the Cordella Sensor in
the right side of the chest. When the
Reader is removed from the Docking
Station it will begin beeping and the light
on the Reader will be solid BLUE.
3. Hold the Reader to the prescribed place
on the chest (that was designated during
training) and listen for a quick series of
beeps which means lock is achieved. The
light on the Reader will blink BLUE in
rapid succession.

12
© 2019 Endotronix® Inc. 100469-00 Rev. 2
4. During the reading, it is important to
remain still and hold the Reader as still
as possible. The light on the Reader will
blink GREEN during a reading. Continue
to breathe normally during the reading.
5. After ~18 seconds the Reader will play a
“Success” tone if the reading worked
properly. The light on the Reader will
become a solid GREEN.
a. In the event the reading obtained was
not good enough due to movement or
improper Reader placement, the
Reader will play a “Fail” tone. The
light on the Reader will blink YELLOW
rapidly. Immediately after, the Reader
will return to “Search” mode
described in step 2.
6. Once a good reading is obtained, the
Reader will be a solid GREEN and will
continue to play the “Success” tone until
it is returned to the Docking Station. The
Reader should always remain docked
unless it is being used or transported.
Submit Results
The tablet screen will either return to the
“Take A Test” screen or you may tap the
“Continue” button, which will appear at the
bottom center of the screen. Tap the “Review
Results” button to advance to the “Test
Summary” screen. The PA pressure reading
will display as taken. Press the “Submit”
button.
NOTE: Once at the “Test Summary”screen, if
all tests have been completed, the application
will automatically submit the measurements
after 30 seconds if no action is taken,
regardless if the test was scheduled or
unscheduled.

13
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Audio & Visual Cues
Reader Audio Cues
Event
Sound
Required Action
Searching for
Sensor
Two descending beeps,
repeating every ~2 seconds
Reposition Reader to
find stronger signal.
Sensor Located
Four quickly ascending,
high pitched beeps,
repeating three times
Great job finding strong
signal! Hold Reader in
place.
Reading in
Progress
Two quickly ascending
beeps, repeating every ~3
seconds
Hold Reader in place
(~18 seconds).
Successful
Sensor Reading
Several quickly ascending,
high pitched beeps
Measurement complete!
Return Reader to
Docking Station.
Failed Reading
Several slowly descending,
low pitched beeps
Reposition Reader to
find stronger signal.
Return to
Docking Station
Successful Reading sound
repeating periodically
Return to Docking
Station. If Reader is
there, check Docking
Station visual cues.
Low Battery
Three quick, low pitched
beeps, repeating every ~10
seconds
When accompanied by
solid yellow light, return
to Docking Station.
Contact
Customer
Service
Three quick, low pitched
beeps, repeating every ~10
seconds
When accompanied by
flashing red light, call
Customer Service.
Reader Visual Cues
Light
Event/Required Action
Solid Blue
Searching for Sensor.
Slowly Flashing Blue
Return to Docking Station.
Rapidly Flashing Blue
Sensor located. Ready to begin reading.
Slowly Flashing Green
Reading in progress. Hold Reader in place until
light becomes solid green.
Solid Green
Successful Sensor reading.
Rapidly Flashing Yellow
Failed reading. Reposition Reader.
Solid Yellow
Low battery. Return to Docking Station.
Rapidly Flashing Red
Contact Endotronix Customer Service.

14
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Light Off
Out of battery. Return to Docking Station.
Docking Station Visual Cues
Light
Event/Required Action
Flashing White
The Reader is being charged.
Solid White
The Reader is fully charged and ready for use.
No Light
When the Reader is docked and no light is
present, the Docking Station is not connected to
a power source. Check that the Docking Station
Power Cord is plugged into both Docking Station
and electrical outlet. If the light remains off,
contact customer service. To turn off audio and
visual cues from the Reader during this time,
put the Reader into Travel Mode (see Traveling
with the myCordella System below).
Traveling with the myCordella Patient Reader and
Docking Station
myCordella PA Sensor System is designed to be portable and should
be taken with you on personal or business travel. Endotronix®
recommends carefully packing the Patient Reader in a carry-on
suitcase by wrapping the Reader in bubble wrap or clothing and
storing in the center of the suitcase. The Docking Station and power
cord should be wrapped separately and also be stored in a suitcase
where it will be under the least amount of stress.
To travel with the Reader, remove the Reader from the Docking
Station and press and hold the travel button on the Reader for several
seconds to initiate Travel Mode. The travel button is a small button
located on the bottom of the Reader, at the base of the handle and
adjacent to the power connector. Depressing the button will disable
the audio and visual cues on the Reader and prevent damage to the
batteries. This same process can be used to Power Off the Reader at
any time. To restart the Reader after travel, press and hold the button
again. If you are unable to charge the Reader during your travels
continue taking your scheduled readings by restarting the Reader,
taking a reading, and powering the Reader back off.

15
© 2019 Endotronix® Inc. 100469-00 Rev. 2
If the button is depressed but the Reader doesn’t respond, the battery
is likely discharged; return the Reader to the Docking Station and
allow the Reader to charge fully.
Ensure you carry your implant ID card with you at all times.
The Reader contains lithium ion batteries. DO NOT
transport in checked luggage.
Wait 5 minutes to take a reading after disabling Travel
Mode to allow the Reader to warm-up.
Contact Us
Questions or concerns regarding setup, use, unexpected operation
or events, and general inquiries can be directed to the contact
information below:
Endotronix® Customer Service
Toll-free: 1-888-512-5595
Support@endotronix.com
Repairing Equipment
To maintain applicable warranties and function, Endotronix® requires
that only authorized personnel perform repairs. There are no user
serviceable parts. Repairs made by unauthorized personnel will
invalidate your warranty. For product warranty information, please
contact Endotronix®. Changes or modifications not expressly
approved by Endotronix may void the user’s authority to
operate the system. Do not dispose of any system components;
contact customer service and follow the RMA procedure below to
return materials to Endotronix. The useful life of the Cordella Sensor
is ten (10) years.

16
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Return Materials Authorization (RMA)
If customer service requests that the equipment be returned, please
follow the directions below.
1) Check off each item on the equipment return list and carefully pack
the equipment in the original shipping box or equivalent with its
original protective packaging materials.
2) Include the RMA number given to you by customer service on the
outside of the shipping container. Ship all equipment and signed
equipment return list to:
RMA #
Customer Service Department, Repairs Endotronix, Inc.
Endotronix, Inc.
815 Ogden Ave
Lisle, IL 60532
USA
Inspection & Cleaning Instructions
Inspect the system regularly. No maintenance is required. If any of the
inspection checkpoints apply, please contact customer service.
Inspection Checklist
oPower cord is not frayed or connected to unauthorized
equipment. If there is a frayed power cord or if the unit is
attached to unauthorized equipment, unplug the unit and
notify customer service to obtain a new one.
oCables are properly attached and in good condition.
oAll accessories are securely attached.
oComponents are not in or near water.
oComponents have not been moved to an unsuitable location.
oIf Reader or Docking Station have been dropped or damaged,
call customer service. Qualified service personnel must inspect
any dropped or damaged units before they are assigned for
use.

17
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Cleaning
oClean the systems components as needed.
oUNPLUG the Docking Station and remove the Reader before
cleaning or disinfecting.
oPut the Reader in “Travel Mode” to minimize audio signals
while cleaning.
oTo clean, wipe the surfaces with a lint-free cloth lightly
moistened in water.
oDO NOT disassemble. Clean only the surfaces of the Reader
and Docking Station.
oDO NOT immerse the Reader or Docking Station in any liquid.
oDO NOT spray liquids directly on the Reader or Docking
Station –use a pre-moistened cloth.
oDO NOT autoclave.
oDO NOT sterilize with ethylene oxide.
MRI Conditions
MRI Information
oYou can have an MRI scan under certain conditions. Talk with
the clinician ordering your MRI scan to ensure that he or she
knows that you have a Cordella PA Sensor.
oIf defined MRI conditions are not followed, there is increased
risk of additional heating or movement of the Cordella Sensor
or of damage to the Cordella Sensor.
oMRI Conditions are described in your physician’s Cordella PA
Sensor System Instructions for Use.
oEnsure you always carry your implant ID card with you.

18
© 2019 Endotronix® Inc. 100469-00 Rev. 2
APPENDICES
Appendix A: Cordella™Pulmonary Artery Sensor System Components
Use only supplies authorized by Endotronix®, as other equipment may
result in increased electromagnetic emissions, decreased immunity to
emissions, or damage to the system. To order supplies, contact
customer service and include the part number and name.
Endotronix, Inc.
815 Ogden Ave
Lisle, IL 60532
USA
Cordella™ Pulmonary Artery Sensor System Component List
100102-00
myCordella Patient Reader
100274-00
myCordella Docking Station
100264-00
Docking Station AC/DC Wall Mount Adapter

19
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Appendix B: Equipment Specifications
myCordella™ Patient Reader
Manufacturer: Endotronix®
CAUTION--Investigational device. Limited by Federal (or United States) law to
investigational use.
Method of measurement: Wireless interrogation of implanted Cordella Sensor
Pulmonary artery pulse pressure maximum range: 40-100 mmHg
Pulmonary artery pressure range at sea level: 0-100 mmHg
Pressure Transducer Accuracy: +/- 7.8 mmHg over full scale range, for
operating conditions between 15°C and 30°C and relative humidity between
6% and 93%.
Patient safety/use: Typical reading time is 30 seconds.
Calibration: At implant and when deemed necessary by a medical professional
Expected service life (of Reader only): One year
Safety standards: Meets all relevant parts of IEC 60601-1 Ed. 3.1
EMC standards: Meets all relevant parts of IEC 60601-1-2 Ed. 4.0
Operating frequency: 12.88-14.12 MHz, 2.45 GHz
Essential performance is maintained for the service life of the product. For
additional information regarding essential performance contact Endotronix
customer service.
Physical
Approximate dimensions
•Width: 6.44 in / 16.35 cm
•Height: 2.0 in / 5.1 cm
•Depth: 5.63 in / 14.3 cm
•Weight: 1.1 lbs / 0.5 kg
Power
Input of 4.2V/16.8V 925mA/250mA
Environment
The Reader may not meet its performance specifications if stored or used
outside the temperature and humidity ranges listed below.

20
© 2019 Endotronix® Inc. 100469-00 Rev. 2
Temperature
•Operation: 15 –30°C (59 –86°F)
•Storage: -10 –55°C (14 –131°F)
Relative humidity
•Operation: 6 –93% (non-condensing)
•Storage: 15 –93% (non-condensing)
EMC: Meets all relevant parts of IEC 60601-1-2
myCordella™ Docking Station
Manufacturer: Endotronix®
CAUTION--Investigational device. Limited by Federal (or United States) law to
investigational use.
Expected service life: One year
Physical
Approximate dimensions
•Width: 5.5 in / 14.0 cm
•Height: 2.5 in / 6.4 cm
•Depth: 5.5 in / 14.0 cm
•Weight: 0.4 lbs. / 181.4 g
Power Cord
Cord length: ~ 8 feet / 2.4 m
AC Power: Wall mount style power supply
•Input of 110-250V , 50-60 Hz
•Output of 5V @ 3A
Manufacturer: SL Power Electronics
Part No: ME20A0503F01
Docking Station
•Input: +5V 3.0 A
•Output: 4.2V/16.8V, 925mA/250mA
Table of contents
Other Endotronix Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Ivy Biomedical Systems
Ivy Biomedical Systems 3150-B Operation manual

Storz
Storz 110 Series manual

Idmed
Idmed WiTOF quick start
Hologic
Hologic Brevera User reference guide

Konica Minolta
Konica Minolta ViZion DR + install guide

NRS Healthcare
NRS Healthcare M48453 Rise Easy Width Adjustable User instructions