eNeura SAVI User manual

1
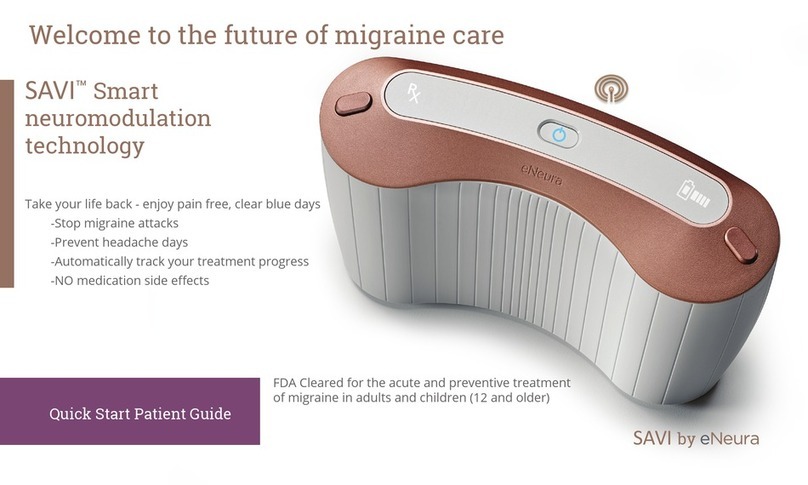
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
SAVI Dual™
Migraine Therapy
Instructions for Use
Prescriber’s Manual
Caution: Federal law (U.S.) restricts this device to sale by or on the order of a physician.

2
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Table of Contents
Before You Begin ......................................................................................................................................................... 3
Intended Use................................................................................................................................................. 4
Warnings and Precautions ............................................................................................................................ 4
Contraindications .......................................................................................................................................... 6
Clinical Trial and Adverse Reactions.............................................................................................................. 8
SAVI Dual ™ ................................................................................................................................................. 11
Getting to Know the Device ...................................................................................................................................... 12
Using the SAVI Dual ................................................................................................................................................... 14
Setting Up the SAVI Dual............................................................................................................................. 14
Recommended Treatment .......................................................................................................................... 14
Preparing for Treatment ............................................................................................................................. 15
Positioning the Device................................................................................................................................. 17
Delivering the Treatment ............................................................................................................................ 18
Renewing the SAVI Dual Prescription by Cellular Network......................................................................... 18
Additional Information.............................................................................................................................................. 19
Traveling with the SAVI Dual....................................................................................................................... 20
Caring for the SAVI Dual.............................................................................................................................. 20
Understanding System Display Messages ................................................................................................................. 21
Troubleshooting ........................................................................................................................................................ 22
Service......................................................................................................................................................... 23
Return Goods Policy.................................................................................................................................... 24
Technical Specifications ............................................................................................................................................ 24
Operating Environment............................................................................................................................................. 24
Storage Environment .................................................................................................................................. 25
Industry Standard Classification.................................................................................................................. 25
REACH and Warning Statement.................................................................................................................. 25
EMC Compliance and Warning Statement................................................................................................................ 26
EMC Guidance............................................................................................................................................. 26
EMC Guidance (continued) ......................................................................................................................... 27
Wireless Connection.................................................................................................................................................. 29
Wireless Connection Troubleshooting........................................................................................................ 29
Glossary of Abbreviations ......................................................................................................................................... 30
Key to Symbols............................................................................................................................................ 30
Medical Device Reporting ......................................................................................................................................... 31
Warranty and Limitation of Liability.......................................................................................................................... 31
Customer Care Contact Information ......................................................................................................................... 31

3
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Before You Begin
This manual provides the information you need to prescribe the eNeura SAVI Dual ™ Migraine Therapy to your
patients. You will see cautions, warnings, and helpful information placed near the related steps. Call Customer
Care if you don’t understand something in this manual.
Please read this entire manual thoroughly before prescribing the SAVI Dual. Review the contraindications,
cautions, warnings and notes regarding the use of the device. As the manufacturer, eNeura cannot and does not
intend to give medical advice. This manual must be available for your reference when discussing the device with
your patients.
eNeura is committed to the service and support of our customers.If there are any questions about the use of the
eNeura SAVI Dual, please contact Customer Care or your local representative at the following:
Manufactured by: eNeura Inc.
101 W. Dickman Street, Ste 900
Baltimore, MD 21230 USA
eNeura Inc.
Corporate Headquarters
101 W. Dickman Street, Ste 900
Baltimore, MD 21230 USA
Tel: +1 408.213.8136
Toll free (USA only): +1 833.499.9300
Fax: +1 877.264.1818
www.eNeura.com
info@eNeura.com
customercare@eNeura.com
eNeura (UK) Ltd.
6th Floor
One London Wall London EC2Y 5EB
United Kingdom
www.eNeura.co.uk
Tel. +44 (0) 20.3356.4889
Fax: +44 (0) 20.7785.8152
customercare@eneura.co.uk
Authorized Representative
Emergo Europe
Westervoortsedijk 60
6827 AT Arnhem
The Netherlands
Tel: +31 (0) 70.345.8570
Fax: +31 (0) 70.346.7299
www.emergoeurope.eu
Australia Sponsor
Emergo Australia
Level 20, Tower II, Darling Park
201 Sussex Street
Sydney, NSW 2000
Australia
www.emergogroup.com
4
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Intended Use
The SAVI Dual (The System) is indicated for the acute and prophylactic treatment of migraine headache in
adolescents (age 12 and older) and adults.
The System is intended for self-treatment and delivers a non-invasive, brief, single pulse of magnetic energy to the
back of the head.This creates a brief electrical current in the brain intended to stop or reduce the effects of
migraine headaches.
The System is a drug-free treatment option that can be used in the home or away from home. The patient should
use the device based on your instructions.After treatment, there are no restrictions.Patients can resume their
normal activities.
WARNING: This device should be used under your continued supervision of the patient.
Instruct the patient to keep the SAVI Dual out of the reach of children.
Safety and effectiveness have not been established in pregnant women and in children under
the age of 12.
The long-term effects of single-pulse transcranial magnetic stimulation are unknown.
Warnings and Precautions
The words WARNING, Precaution and NOTE have special meanings in this manual. Read them throughout the
manual to ensure the safe and effective use of your SAVI Dual.
WARNING: A WARNING tells you that the personal safety of the patient may be involved.
Ignoring a WARNING could result in injury to the patient. WARNINGS in the manual are shown in
an orange box.
Precaution: A Precaution means that exact steps must be followed to prevent damage to the
product.Precautions in the manual are shown in a purple box.
NOTE: ANOTE gives special information to ease product use or to explain important information.
NOTES in the manual are shown in a dashed box.

5
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
WARNING: The SAVI Dual should be used only under your continued supervision of the
patient.Advise the patient that the System has been prescribed only to be used by them.
Patients must be aware that the device is non-transferable and that failure to follow this
instruction could result in minor to serious injury, including death, to the patient and the
unintended user.
Inspect the System for any signs of damage before use. Advise the patient to not use it if it is
cracked or wet.If you or your patient suspect damage to the device, call eNeura Customer
Care at: +1 833.499.9300 option 1 for assistance.
Do not operate the System in or near an area where explosive gases are being used or have
been used. Do not operate near gasoline or natural gas.
Do not operate the System in or near the presence of a FLAMMABLE ANESTHETIC MIXTURE
WITH AIR or WITH OXYGEN or NITROUS OXIDE.
Risk of electrical shock. Do not open the System. There are no parts that can be serviced or
replaced by the user. High voltage may be present.
Risk of electrical shock. Do not allow the System or power cords to get wet. Quickly wipe up
spills on or near the SAVI Dual. Do not use the System in or near water. For example, do not
use while in the bathtub or shower, in the rain, or while standing in water or on a wet surface.
Instruct your patients that the System should not be used if the cause of the headache is
illness, underlying pathology, trauma or overuse of medication.
TMS treatments should not be used in patients with suspected or diagnosed epilepsy or a
personal or family history of seizures. Prior to prescribing eNeura SAVI Dual, carefully review
your patient’s history for a family history of epilepsy or seizures; patient history of a head
trauma or head injury or a current prescription for any medication such as tricyclic
antidepressants, neuroleptic agents, or other drugs that lower the seizure threshold.
TMS treatment should not be used if the patient has a history of stroke.
The System has not been demonstrated as safe or effective when treating cluster headache.
TMS treatments should not be used on patients who use a wearable cardioverter defibrillator
(WCD).
The long-term effects of chronic magnetic stimulation are unknown.
Transcranial magnetic stimulation should only be applied to the back of the head as described
in the “Using the Device” section of this manual.
Instruct your patient that the eNeura SAVI Dual should not be used to stimulate over the front
of the neck or mouth.Severe spasm of the laryngeal and pharyngeal muscles may occur, and
the contractions may be strong enough to close the airway or cause difficulty in breathing.
Instruct your patient that the eNeura SAVI Dual should not be used to stimulate over the upper
side of the neck.Stimulation of the carotid sinus nerves, particularly in patients with a known
sensitivity to the carotid sinus reflex, could result in a sudden drop in blood pressure, slowing
of the heart or loss of consciousness.
Instruct your patient that the eNeura SAVI Dual should not be used to stimulate the chest or
back. The induction of electrical current into the heart may cause cardiac arrhythmias.

6
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Contraindications
WARNING: Failure to follow the restrictions listed below could result in serious injury or
death.
The SAVI Dual creates a very strong single-pulse magnetic field. The System should be used only under your
continued supervision of the patient. Advise the patient that the device has been prescribed only for use by them.
The System may not be used in patients who have metals, conductive materials, or metal-containing implants in
their head, neck or upper body. Metals and conductive materials can be affected by a magnetic field. Discuss this
thoroughly with your patient before prescribing the device.
Patients MUST NOT use the System if they have a cardiac pacemaker, vagus stimulator (VNS) or other implanted
neurostimulator, implanted cardioverter defibrillator (ICD) or any implanted medical device that stimulates the
body or uses any signal from the body.Do not prescribe the device to these patients.
If your patient has implants that are affected by a magnetic field, they MUST NOT be prescribed the use of the
System. Failure to follow this restriction could result in serious injury or death. Examples of such implants
include:
•Aneurysm clips or coils
•Cochlear implants
•Cerebral spinal fluid shunts
•Bullets or pellets lodged in the head
or upper body.
•Metal plates, screws, staples or
sutures in skull, neck, shoulders,
arms or hands
•Radioactive seeds
•Magnetically programmable shunt valves
•Stents
•Filters
•Metallic artificial heart valves
•Facial tattoos with metallic ink
•Electrodes
Dental implants, fillings or other dental appliances are okay and are not affected by the device.
Advise your patient to inform you if following your prescription, they will require an intervention that includes
implantation of any device or other object. In such case, verify whether the implant will contain any conductive
substances. If so, instruct your patient to discontinue use of the System.
7
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
WARNING: Instruct your patient that the eNeura SAVI Dual should not be used while driving,
operating machinery or during any activity in which involuntary muscle contractions may put
the user at risk of injury.
Instruct your patient to stay at least 2 feet (0.6 meter)from others when using the System.
The System could be harmful to anyone with an electronic implant such as a pacemaker.
Anyone with a hearing aid or cochlear implant may hear an audible click.
The device could be disrupted by RF-emitting equipment including wireless home network
devices, mobile phones, cordless telephones and their base stations and walkie-talkies. See
“EMC Compliance and Warning Statement” section for additional information on preventing
unwanted interference.
•Precaution: Instruct your patient to keep the System away from other electronic devices that
depend-on (receive) or radiate (transmit) radio frequency energy when it is powered on.
•The operation of the System may be impaired when operated near home devices such as
wireless network routers, mobile phones, cordless telephones and their base stations and
walkie-talkies. Keep the SAVI Dual device at least 2 feet (0.6 meter) from these devices when it
is powered on and in use.
•Instruct your patient to keep credit cards, audio/video tapes, computers, computer disks, flash
memory sticks, cell phones, personal digital assistants (PDAs), MP3 players, headphones,
digital cameras, portable glucose meters and other electronic devices or electronic storage
media more than 2 feet (0.6 meter) away from the System when it is in use.
•Instruct your patient to keep any loose metal objects such as eyeglasses, keys, coins, jewelry,
watches, and hair clips more than 2 feet (0.6 meter) away from the System when it is in use.
•Instruct your patient to keep wearable medical devices such as insulin pumps, medicinal
pumps, monitors, bone grow stimulators and Transcutaneous Electrical Nerve Stimulator
(TENS) devices more than 2 feet (0.6 meter) away from the System when it is in use.
•Safety and effectiveness have not been established in pregnant women and in children under
the age of 12.
•Caution should be used for patients with suspected or diagnosed heart problems.
•The System is only intended to be serviced or maintained by the manufacturer. Do not
attempt to open the device.The warranty may be invalidated. If the device is opened, contact
eNeura Customer Care at: +1 833.499.9300 option 1.
•Instruct your patient to keep the System out of the reach of children.
•Side effects can include minor dizziness, nausea, vomiting, application site tenderness, muscle
spasm, headache and migraine.
•Special precautions regarding Electromagnetic Compatibility (EMC) are required when
installing and using the System. Portable and mobile communications devices can affect
proper operation of the System. See “EMC Compliance and Warning Statement” section of the
Instructions for Use for more information.
•Advise your patient that the eNeura SAVI Dual should be installed and put into service
according to the EMC information provided in this Physician Manual.
•Advise your patient to see “EMC Compliance and Warning Statement” section of the Patient
Manual for more information.

8
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Clinical Trial and Adverse Reactions
Summary of Clinical Data:
eNeura sTMS Post-Market Observational U.S. Study of Migraine(“ESPOUSE”).
A Multi-Center, Prospective, Non-Randomized, Single Arm, Open Label, Post-Market, Observational Study to
evaluate the Use of the eNeura, sTMS System in reduction of Migraine Headache symptoms.
eNeura completed a prospective, single-arm, non-randomized NSR clinical study in United States centers of
excellence designed to establish safety and effectiveness for use of the eNeura device for migraine headache
treatment and prevention. The effectiveness control for this study population was a performance goal derived
from the literature of patients with similar migraine frequency with respect to the number of reduced headache
days in 12 weeks in placebo or sham patients of randomized clinical trials. Baseline medication and symptoms
were recorded for 28 days via patient diary. Patients were instructed to treat daily using the following protocol
with no change in preventive medication and to complete Month 1, 2, and 3 diaries.
Six patients reported using prophylactic medications at baseline (2.8%), of these, 5 were using topiramate and
propranolol.
The efficacy of the eNeura device for prophylactic treatment is based on the result of an open label study. In open label
studies, bias may affect the result. Additionally, open-label studies may introduce placebo rates of 10-25%. This is
consistent with placebo rates reported in Randomized Controlled studies for migraine prevention.*
ESPOUSE Treatment Protocol:
Treat with 4 Pulses each morning and evening:
2 consecutive pulses wait 15 minutes and repeat the 2 consecutive pulses.
Additionally, the patient may treat an acute attack with:
3 sequential pulses (early) at the onset of migraine pain
Wait 15 minutes.
If needed treat with additional 3 pulses
Wait 15 minutes.
If needed treat with additional 3 pulses
Patients could rescue with acute medication 30 minutes after the first three pulses are delivered.
A total of 263 subjects were consented between December 2014 and March 2016. 229 of these completed a
Baseline Diary and 220 were confirmed by the sites to be eligible for participation. There were 217 subjects that
were assigned an active eNeura device and these subjects comprise the Safety Data Set. There were 179 subjects
who began treatment and completed a Month 1 treatment diary, but 47 of these subjects did not meet the
minimum requirement of at least 4 days with moderate to severe headache pain for at least 4 hours at baseline.
Thus, 132 of these subjects complied with the protocol requirements based upon headache day definition. This
was the Full-Analysis Data Set (FAS) described below. There were 117 of these subjects that went on to finish
treatment and completed both baseline and Month 3 diaries. This was the Completed Cases data set (CC). Of
these subjects, 95 complied with the protocol instructions regarding use of the device. This was the Per Protocol
(PP) data set.
*Macedo A, Banos JE, Farre M. Placebo response in the prophylaxis of migraine: a meta-analysis. Eur J Pain 2008; 12;68-75
9
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Primary End Point:Study results showed statistically significant reduction in migraine headache days of 2.8 days
(from a baseline mean of 9.1 days) (FAS), P<0.0001; 2.8 days (from a baseline mean of 8.9 days) (CC) P<0.0001, and
3.0 days (from a baseline mean of 9.1 days) (PP) P<0.0001.
First Secondary Endpoint: Of the 117 subjects in the CC group,
54 (46.15%) had a 50% or greater reduction in headache days
at three months, P<0.0001.
Second Secondary Endpoint: Reduction in acute medication days.
Reduction in Acute Medication Days in in Three Months
Endpoint
Baseline
Mean, (SD) N
Med (Min,
Max)
Change
Mean, (SD)
N
Med (Min,
Max)
95%
Confidence
Interval
t-statistic
P-value
Acute
Medication
Days (CC)
9.95 (5.63)
117
10.0 (0, 28)
-2.93 (5.24)
117
-2.0 (-23, 10)
(-3.89, -1.97) -6.05 <0.0001
Acute
Medication
Days (PP)
10.38 (5.76)
95
10 (0, 29)
-3.18 (5.45)
95
-3 (-23, 9)
(-4.29, -2.07) -5.69 <0.0001
Third Secondary Endpoint: The reduction from baseline in the HIT6 impact
questionnaire quality of life (HIT-6) improvement of 3.1 (CC) and 3.6 (PP).
Reduction in HIT 6 Score at Three Months
Endpoint
Baseline
Mean, (SD) N
Med (Min,
Max)
Change
Mean, (SD)
N
Med (Min,
Max)
95%
Confidence
Interval
t-statistic
P-value
HIT6 (CC)
63.85 (4.56)
117
64.0 (50, 76)
-3.10 (6.42)
114a
-2.0 (-25, 11)
(-4.29, -1.90) -5.15 <0.0001
HIT6 (PP) 64.04 (4.56) 95
64 (52, 76)
-3.63 (6.79)
94b
-2 (-25, 11)
(-5.02, -2.24) -5.18 <0.0001
Additional Disability measure MSQOL.
Increase in MSQOL Domain Scores at Three Months
Endpoint
Baseline
Mean, (SD) Na
Med (Min,
Max)
Change
Mean, (SD) Na
Med (Min,
Max)
95%
Confidence
Interval
t-statistic
P-value
MSQOL RR
(CC)
42.49 (19.69)
116
42.86 (0,
91.43)
16.12 (24.54)
113
17.14 (-48.57,
80.00)
(11.55, 20.70) 6.98 <0.0001
MSQOL RP
(CC)
59.45 (23.51)
116
62.5 (0, 100)
12.55 (25.23)
113
10.0 (-50,
100)
(7.85, 17.25) 5.29 <0.0001
MSQOL EF
(CC)
48.16 (27.94)
112
46.67 (0, 100)
16.40 (29.01)
113
13.33 (-40,
100)
(10.99, 21.81) 6.01 <0.0001

10
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
MSQOL RR
(PP)
40.09 (19.85)
94
40 (0, 91.43)
18.97 (24.90)
93
20 (-48.57,
80)
(13.85, 24.10) 7.35 <0.0001
MSQOL RP
(PP)
56.54 (24.30)
94
60 (0, 100)
14.84 (26.47)
93
10 (-50, 100)
(9.39, 20.29) 5.41 <0.0001
MSQOL EF
(PP)
44.26 (27.89)
94
40 (0, 100)
19.43 (29.22)
93
20 (-40, 100)
(13.41, 25.44) 6.41 <0.0001
Fourth secondary endpoint: The reduction from baseline in the
days with headache for more than 4 hours with any pain
intensity.
Reduction in Headache Days Three Months
Endpoint
Baseline
Mean, (SD) N
Med (Min,
Max)
Change
Mean,
(SD) N
Med (Min,
Max)
95%
Confidence
Interval
t-
statistic
P-value
Headache
Daysa(CC)
10.58 (4.33)
117
10.0 (4, 24)
-3.16
(5.21) 117
-4.0 (-22,
9)
(-4.12, -
2.21) -5.25 <0.0001
Headache
Daysa(PP)
10.79 (4.32)
95
10 (4, 24)
-3.28
(5.16) 95
-4 (-22, 9)
(-4.34, -
2.23) -5.01 <0.0001
Safety: Approximately 29% of the 217 subjects included in the
Safety Dataset reported experiencing at least one adverse event
in this study. No subject had events that could be determined
to be serious adverse events. None of the events required
treatment. Adverse events as described below are the same as
those reported in previous studies.
Adverse Events Reported in the ESPOUSE Study (greater than 2%)
Adverse Event
x/n (%)
95% LCL,
95%UCL
Reported Relationship to Device
Any
62/217
(28.57)
22.66, 35.08
19 Not related, 27 Possibly, 7 Probably,
5 Definitely, 4 Not Specified
Headachea
5/217
(2.30)
0.75, 5.30 1 Not related, 4 Possibly
Scalp Discomforta
5/217
(2.30)
0.75, 5.30 1 Possibly, 4 Probably
Tinglinga
7/217
(3.23)
1.31, 6.53
2 Possibly, 3 Probably, 1 Definitely,
1 Not Specified
Light Headednessa
8/217
(3.69)
1.61, 7.14 1 Not related, 6 Possibly, 1 Probably
Discomfort from
Noisea
5/217
(2.30) 0.75, 5.30 Not related, 2 Possibly, 2 Definitely
Dizziness
6/217
(2.77)
1.02, 5.92 5 Possibly, 1 Definitely
Ringing in Ears
(Tinnitus)
7/217
(3.23) 1.31, 6.53 1 Not Related, 6 Possibly
Worsened Headache
Pain
5/217
(2.30) 0.75, 5.30 3 Possibly, 2 Not specified

11
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
SAVI Dual ™
The complete SAVI Dual includes the SAVI Dual Patient Quick Guide, part number LBL-0195, and the following
items:
The battery-powered, rechargeable device
Carrying bag for SAVI Dual and its AC adapter battery charger
Note: The carrying bag is part of the packaging for shipping
the SAVI Dual device. Please place the device into the bag prior to
boxing the device for return/exchange.
Battery Charger 12V DC 1.5A 18 watts
(reorder no.DWG-0505)
NOTE: Do not discard the box or packing materials.
They will be required if you need to return this
product.

12
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Getting to Know the Device
A. Power Button In the center of the panel on the top of the System.Press the power button to turn
the device on and off.
B. Power Indicator LED light on the top of the System inside the Power button. Static white illumination
shows the device is on and ready.Pulsing blue illumination indicates device is connecting to the cellular network
to transfer data or upload prescription data.
C. Treatment Progress Indicator LED light around the power button on the top of the System, shows
status as it prepares for treatment.
D. Prescription Status Indicator Located on left side on the top of the System. Confirms a valid prescription is
available and shows the status of the prescription: White illumination indicates Rx is active; static orange
indicates Rx will expire soon; orange with orange Power Button indicates Rx has expired.
E. Contact eNeura Customer Care Action required. Call Customer Care.
F. Temperature warning The device temperature is not in range for safe use.
G. Lock Indicator On the right of the Power button.Indicator is visible when device is turned on if the security
lock switch is enabled.
H. AC Adapter On the right of the Power button. Indicator is lit when the AC adapter is connected to the
device.
I. Battery Capacity On right side on the top of the System. Indicates whether battery power is enough to
allow treatment. White illumination indicates battery power is available; orange illumination indicates battery
power will soon be or has been exhausted.
J. Treatment Buttons On the right and left edge of the panel on the top of the device. Press one or both buttons
to deliver a treatment.
Read “Understanding System Display Messages” for more information.
A
B
C
F
G
H
I
J
D
E

13
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
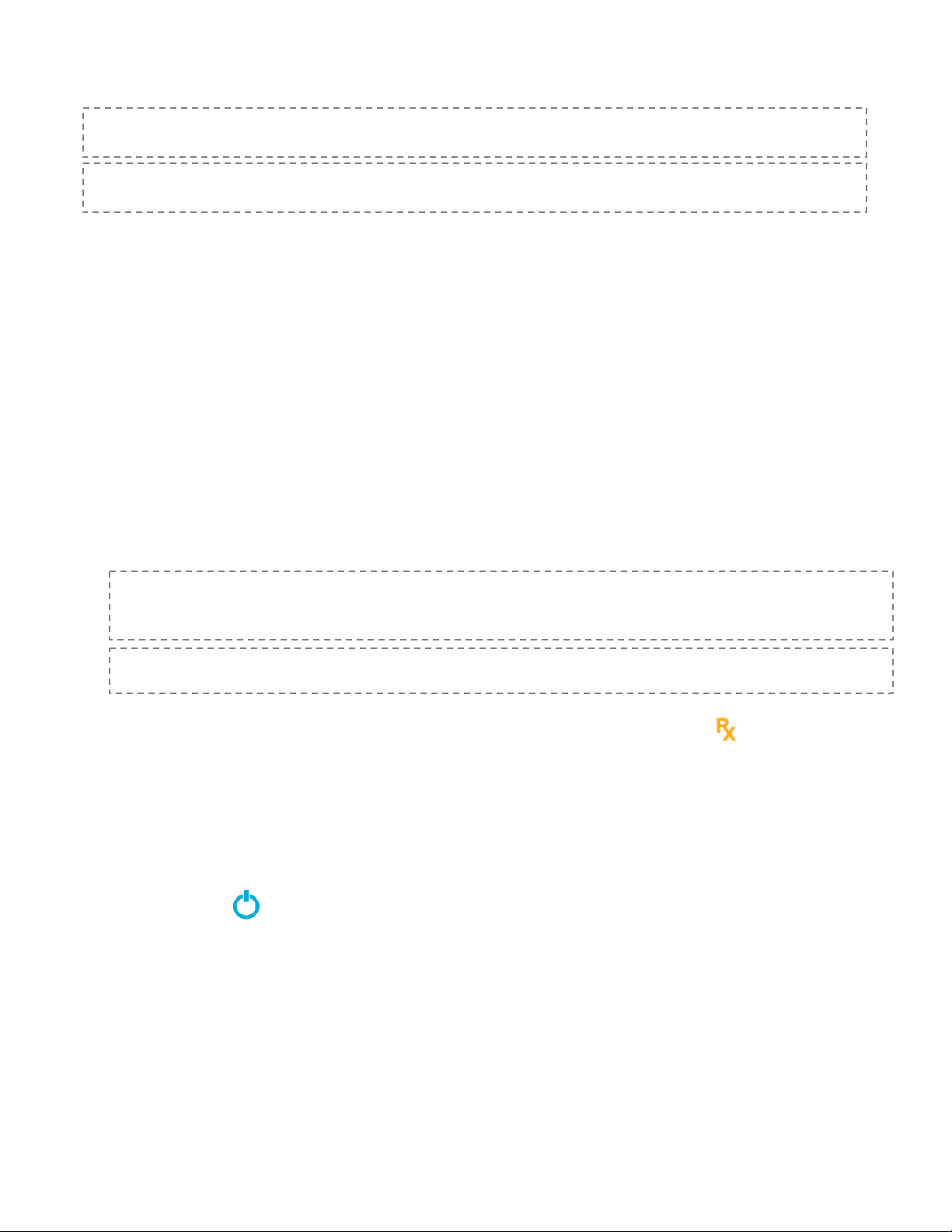
Battery Charger (Power Supply) and micro-SIM Rx card Ports
K. Lock ON/OFF Switch –Located behind accessory port door, on the left. Slide the switch to the left or right to
unlock or lock the device.
L. Volume ON/OFF Switch – Located behind accessory port door, on the right. Slide the left or right to turn the
sound on or off.
M. Battery Charger Port – Located behind accessory port door, in the center. Recharge the batteries by plugging
the AC battery charger, DWG-0505, into this port.
AC battery charger 12V DC
1.5A 18 watts
(reorder no.DWG-0505)
Plug that connects to
the System.
N. Micro-SIM Rx card port – Located on the back of the System beneath the Accessory Door. Insert the micro-SIM
RX card into this port. A micro-SIM card is only required if SAVI Dual prescription is NOT renewed by wireless
cellular network.
•Precaution: Use only eNeura-supplied accessories with the SAVI: Battery charger (reorder no.
DWG-0505) and micro-SIM Rx card.
M
N
L
K

14
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Using the SAVI Dual
•Precaution: Instruct your patient that this manual must be completely read and understood
prior to use of the SAVI Dual. Improper use may cause personal injury and damage to the
System and may void the warranty.
•Instruct your patient to become familiar with the functions and features of the device prior to
use.
Setting Up the SAVI Dual
Instruct the patient to be prepared to treat their migraine when needed.Instruct the patient to set up the SAVI
Dual as soon as it arrives and keep the device charged and ready to treat.
NOTE: Instruct the patient to use only as directed.
Instruct the patient to use the device as follows:
Remove the System and the battery charger from the box.
NOTE: Instruct the patient not to discard box or packing materials. They will be required if there
is a need to return this product.
The device should arrive ready to use. If it is not ready, plug the battery charger into a standard wall outlet.
Connect the other end of the battery charger to the port on the device. Charge the battery for about 4 to 6
hours.
Treatment delivery is not available during battery charging.
If the device is on while charging, the AC adapter symbol and the battery capacity symbol will be lit.
Unplug the battery charger when the device is fully charged. A fully charged battery should deliver at least 12
treatments.
Keep the accessory door closed when not in use.
Store the device and its battery charger in a cool, dry place, away from excessive dust and direct sunlight.
•Precaution: Only use the battery charger (DWG-0505) included with the SAVI Dual. Contact
eNeura Customer Care if you have any questions.
Recommended Treatment
The treatment with the SAVI Dual should be performed per your instructions as the prescribing physician. Read
the instructions below prior to operating the device. When discussing the treatment with the patient, emphasize
the importance of following your instructions, the instructions in the Patient Manual and adhering to the
prescribed treatment regimen. Review the instructions below with your patient.
Prevention
Treat with 4 pulses each morning and evening
Acute
4 sequential pulses at the earliest indication of an attack
After a brief pause, if the patient does not experience symptom relief, they can treat again as needed.
NOTE: Instruct your patient to become familiar with the System and how to use it before they
experience a migraine.
15
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Preparing for Treatment
Consult with your patient about their specific condition and the best timing of treatment with SAVI Dual .
•Precaution: Instruct your patient to keep the System away from other electronic devices that
depend on (receive) or radiate (transmit) radio frequency energy when it is powered on.
•The operation of the System may be impaired when operated near home devices such as
wireless network routers, mobile phones, cordless telephones and their base stations and
walkie-talkies. Keep the SAVI Dual device at least 2 feet (0.6 meter) from these devices when it
is powered on and in use.
•The System emits a strong magnetic pulse that may interfere with the operation of common
home electronic devices such as radios, televisions, wireless network routers, mobile phones,
cordless telephones and their base stations and walkie- talkies, if they are not installed and
used in accordance with the manufacturer’s instructions. There is no guarantee that
interference will not occur in a particular installation.If the System causes interference with
other devices, try to correct the matter by:
•Reorienting or relocating the device receiving the interference.
•Increase the separation distance between the System and the device.
•Connect the System to an AC outlet on a circuit different from that to which the other
device is connected.
Use the System in a comfortable, seated position. Remove the System from its storage location.
WARNING: Inspect the System for any signs of damage before use.Advise your patient to
not use if it is cracked or wet.If you suspect damage to the device, call eNeura Customer
Care at +1 833.499.9300 option 1 for assistance.
The System needs to be in direct contact with the back of your head to work properly.Before treatment, remove
any hat, head covering, or hairpiece that covers the back of your head and undo any braids, ponytails or buns.
WARNING: Remove any personal medical devices such as insulin pumps or other medical
pumps and bone growth stimulators.
Press the power button
Watch the LED track light progress around the power button:
It takes 30-60 seconds to prepare the System for treatment.
As the device prepares for treatment, each segment will light up to indicate progress.
When the system is ready, the device lights up all 6 segments and sounds an audio tone:
Once the System is ready, you have 45 seconds to position the device and deliver treatment.
16
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
WARNING: Instruct your patient to stay at least 2 feet (0.6 meter)from others when using
the SAVI Dual. This device could be harmful to anyone with an electronic implant such as a
pacemaker.
Remove any personal medical devices such as insulin pumps, other medicinal pumps, bone
growth stimulators, Transcutaneous Electrical Nerve Stimulator (TENS) devices and hearing
aids.
Do not use the System on patients who use a wearable cardioverter defibrillator (WCD).
Instruct your patient that the System should not be used while driving, operating machinery
or during any activity in which involuntary muscle contractions may put you at risk of injury.
Risk of electrical shock: Do not allow the System or power cords to get wet. Advise your patient
to quickly wipe up spills on or near the device. Do not use the device in or near water. For
example, do not use while in the bathtub or shower, in the rain or while standing in water or
on a wet surface.
Do not operate the System in or near an area where explosive gases are being used or have
been used. Do not operate near diesel fuel, gasoline or natural gas.
Do not operate the System in or near the presence of a FLAMMABLE ANESTHETIC MIXTURE
WITH AIR or with OXYGEN or NITROUS OXIDE.
Instruct your patient that the System should not be used if the cause of the patient’s headache
is illness, trauma or excess medication. Consult your doctor if you are unsure.
Do not use the System in patients with suspected or diagnosed epilepsy. Consult your doctor
before using the System if a family member has epilepsy. Prior to prescribing the SAVI Dual,
carefully review your patient’s history for a family history of epilepsy, seizures, head trauma
or head injury, or if the patient is currently using any medication such as tricyclic
antidepressants, neuroleptic agents or other drugs that lower the seizure threshold.
Do not use the System if the patient has a history of stroke.
Caution should be used for patients with suspected or diagnosed heart problems.
•Precaution: Keep the SAVI Dual away from metal or conductive objects, medical devices and
magnetic media when it is powered on.
•When activated, the System emits a strong magnetic pulse and may interfere with other metal,
electronic or magnetic products within 2 feet (0.6 meter). A person with a hearing aid or
cochlear implant may hear a click when the System is activated.
•The operation of the SAVI Dual System may be impaired when operated near home devices
such as wireless network routers, mobile phones, cordless tele- phones and their base stations,
and walkie-talkies. Keep the SAVI Dual device at least 2 feet (0.6 meter)from these devices
when it is powered on and being used.
oInstruct your patient to move credit cards, audio/video tapes, computers,
computer disks, flash memory sticks, cell phones, personal digital assistants
(PDAs), MP3 players, headphones, digital cameras, portable glucose meters
and other electronic devices or electronic storage media more than 2 feet (0.6
meter) away from the System.
oInstruct your patient to remove all metal objects from your head, face, neck, arms and
hands.Remove eyeglasses, hearing aids, removable metallic orthodontic appliances,
hair clips and earphones and move them more than 2 feet (0.6 meter) away from the
System.
oInstruct you patient to remove any loose metal objects from your shirt pockets such as
keys, coins, clips, pens and mechanical pencils and move them more than 2 feet (0.6
meter) away from the System.
17
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
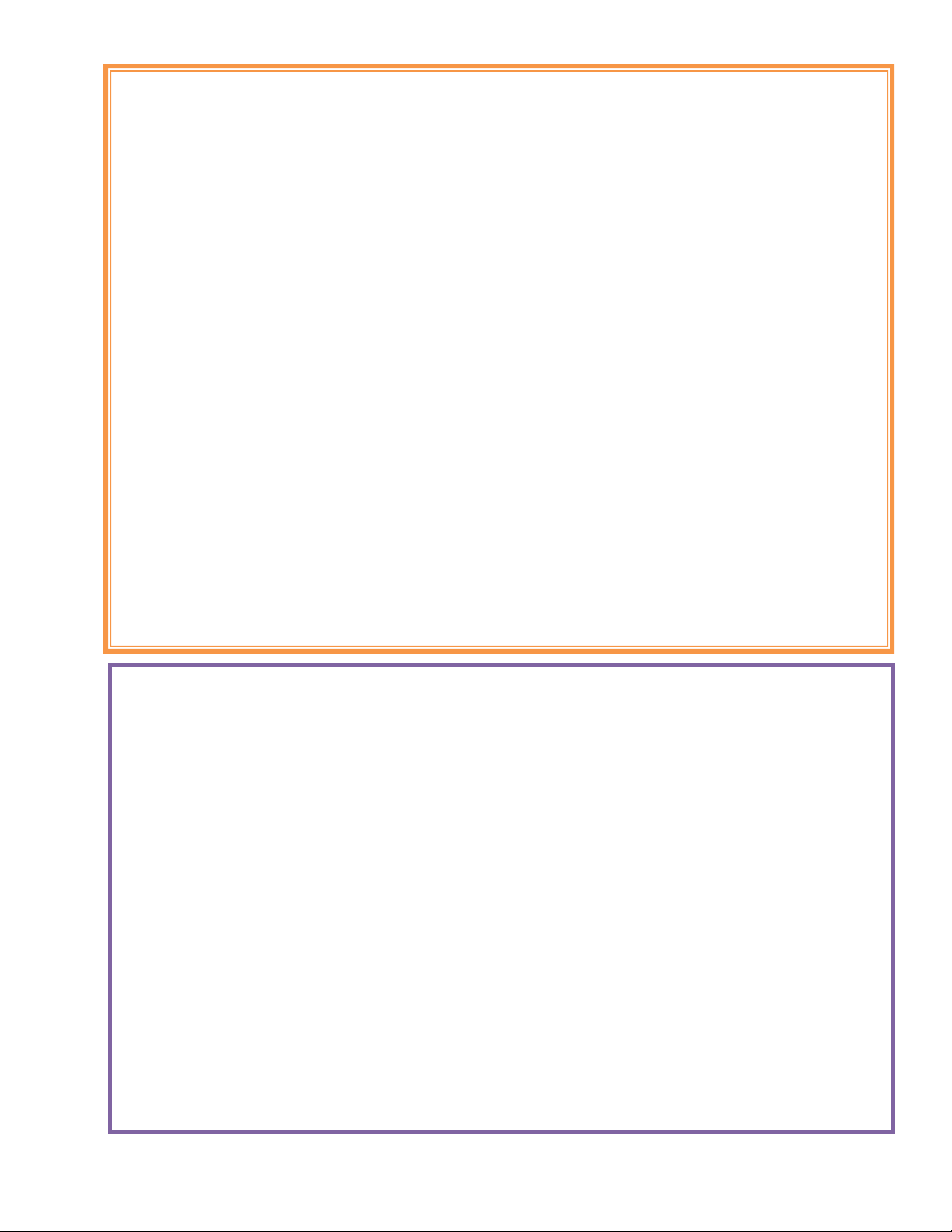
Positioning the Device
With the SAVI Dual in front of the patient: The treatment buttons should be facing up and the accessory door
should be facing away from the patient. The patient places both hands on the sides of the device with thumbs
on the treatment buttons.
The patient lifts the System over their head. The device should be placed so that it comfortably and naturally
cradles the base of the skull.The patient should hold it firmly against their head.
The curved surface positions the device to fit comfortably against the head and optimize pulse delivery.
WARNING: Transcranial magnetic stimulation should only be applied to the back of the head
as described in the “Positioning the Device” section of this manual.
Instruct your patient that the System should not be used to stimulate over the front of the
neck or mouth.Severe spasm of the laryngeal and pharyngeal muscles may occur.
Contractions may be strong enough to close the airway or cause difficulty breathing.
Instruct your patient that the System should not be used to stimulate over the side of the
neck.Stimulation of the carotid sinus nerves, particularly in patients with a known sensitivity
to the carotid sinus reflex, could result in a sudden drop in blood pressure, slowing of the heart
or loss of consciousness.
Instruct your patient that the System should not be used to stimulate the chest or back.
Electrical current into the heart may cause cardiac arrhythmias.
NOTE: If the device detects an error when it is on, a symbol is displayed. See the “Understanding
System Display Messages” and “Troubleshooting” sections of this manual.
NOTE: The System is a handheld device that comes in direct contact with skin.The housing of
the device is made of polycarbonate, a plastic common in consumer medical products, and poses
no handling risk.
18
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Delivering the Treatment
NOTE: Two hands are needed to stabilize the device, but only one treatment button
needs to be activated to deliver treatment.
NOTE: The device shuts itself off if treatment is not delivered within 45 seconds.Press
the power button to turn the device back on and restart the treatment procedure.
Once the device is in place and the patient’s thumbs are on the treatment switches, press one or both
switches and hold for at least 2 seconds.
The device emits a soft, audible click as the treatment is delivered.
After the patient has successfully delivered the first pulse, remove the device from the patient’s head.
The LED light on the POWER button softly pulses to show that treatment is completed. The device is now
ready for another pulse. The patient may select to treat again by pressing the power button.
Instruct the patient to repeat the treatment as prescribed, Positioning the Device.
After delivering a pulse and 30 seconds of inactivity, the device will attempt to connect to a cellular network,
transfer data and will then power off.
Delivery of several pulses in a row may cause the device’s surface temperature to exceed 105.8°F (41°C). The
surface may get as warm as 118.4°F (48°C). Let the device cool for 15 minutes. Try again.
Renewing the SAVI Dual Prescription by Cellular Network
NOTE: The System is intended for use under your care of the patient. For more information on
renewing a patient’s prescription, please contact eNeura Customer Care. An active prescription
is required.
NOTE: The System only delivers treatments if the prescription has not expired.
The device will display an ORANGE warning light when the prescription is about to expire:
Advise your patient to contact eNeura Customer Care to request a refill if they are not enrolled in the SAVI
Dual monthly subscription program.
After payment is received for the prescription, Customer Care will initiate reactivation of your patient’s device
via cellular network.
To download the new prescription, advise your patient to ensure the System lock switch is OFF and the AC
adapter is not connected to the device.
Press the Power Button on the device. After approximately 30 seconds, pulsing blue illumination on the
Power Button indicates the device is attempting to connect to the cellular network.
After the device has successfully connected to a network, the new Rx data will transfer, and the ORANGE Rx
warning light will become white again. The patient may resume use of the device.
Advise your patient to contact eNeura Customer Care if they reside in a low or no-cell service area or if their
device does not connect to the cell network. A micro-SIM card can be provided to renew the prescription.
19
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Additional Information
Battery Capacity Indicator
The SAVI Dual has rechargeable batteries.The battery capacity indicator on the device shows how much charge is
remaining in the battery.eNeura recommends that patients keep their device’s battery charged so that it is always
ready when needed. Typically, a fully charged battery can deliver 18 treatments. The battery pack life is roughly a
minimum of 100 charge cycles.
Recharging the Battery
To recharge the battery, push the round plug on the battery charger into the battery charger port on the back
of the device.Plug the battery charger into a standard wall outlet.
The AC adapter must be disconnected to allow the device to connect to a cellular network. Wait for the device
to shut itself off before connecting the AC adapter.
The AC adapter, power button and battery power indicator symbols will be lit for approximately 10 seconds
when the AC adapter is connected to the device. After 10 seconds, the lights will shut off while the AC adapter
continues to charge the battery pack.
Treatment while recharging is disabled for safety. To use the device for treatment, unplug the battery charger
and proceed with treatment.
WARNING: The battery charger cable may present a strangulation or trip hazard if the
System is left unattended around infants and small children. Recharge the System’s battery
in a safe location, away from foot traffic and children.Store the battery charger in a safe
place when not in use.
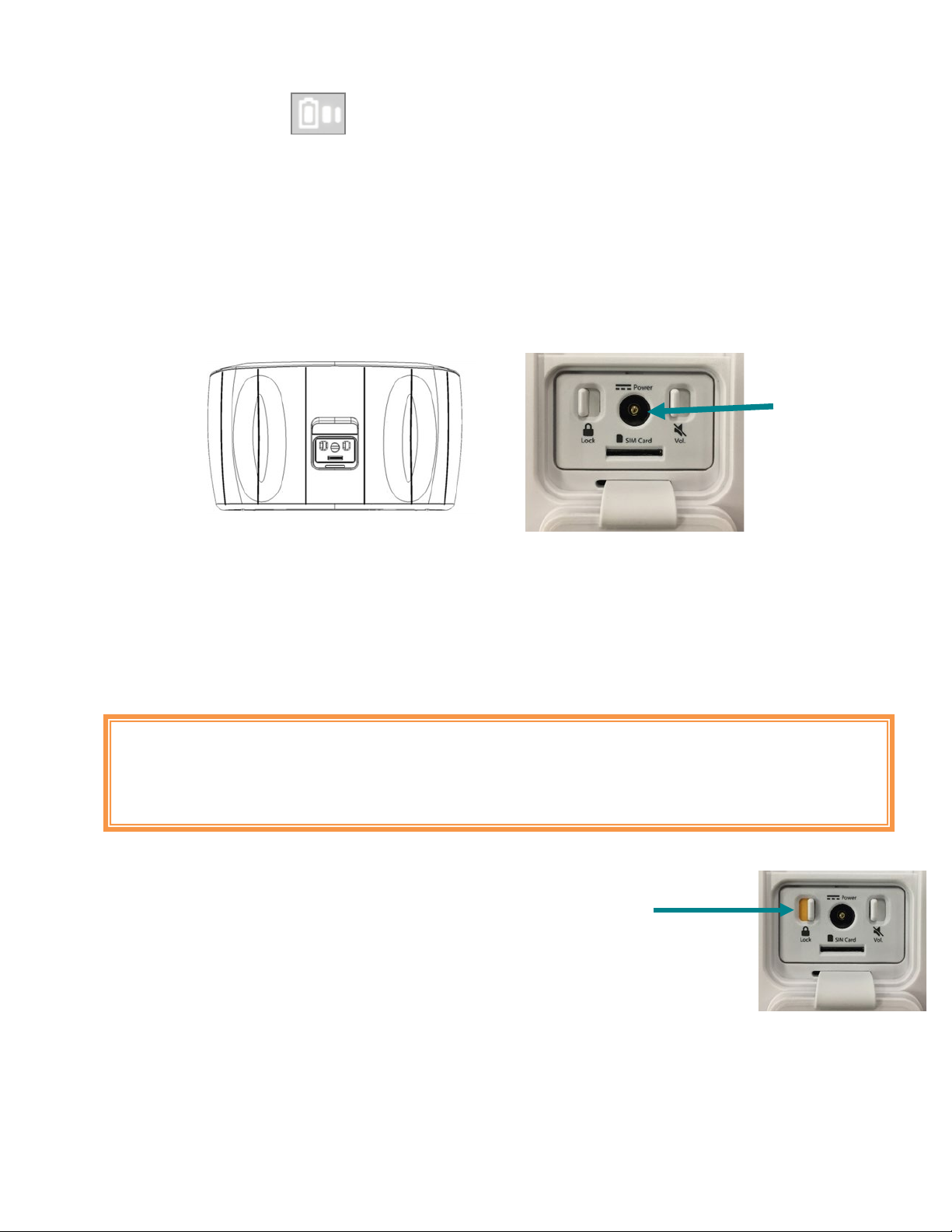
Locking the System/Airplane Mode
The System lock switch is located beneath the Accessory door.
The System lock may be controlled by sliding the switch to the desired position (ON or OFF).
When the System is locked, the device will prevent others from using it by disabling
treatment functionality.
The System lock switch must be OFF to allow the device to connect to a cellular network. Wait for the device
to shut itself off before you lock it.
Activating the System lock switch places the device in Airplane Mode. Place the System in
Airplane Mode whenever you are in a location where use of cellular devices is discouraged or forbidden.

20
SAVI Dual Migraine Therapy Prescribers Instructions for Use Manual
Adjusting the Audible Tone
The System sounds an audible tone when the device is ready to deliver a treatment.
The switch to control the audible tone is located beneath the Accessory door.
Slide the switch to the desired position (ON or OFF).
Traveling with the SAVI Dual
The SAVI Dual may travel with your patient.Place the device in Airplane Mode as explained on page 18 (see
“Locking the System/Airplane Mode”) and pack it securely to avoid damage during travel. Before using the
unit after traveling, check it for any damage to ensure it appears to be working correctly before use.
Whenever possible, pack the SAVI Dual in a carry-on rather than in checked luggage.
At security, advise your patient to remove the SAVI Dual from their carry-on, place it in a bin and, before it
goes through x-ray, notify TSA or Airport Security that it is a medical device. This will speed up the security-
check process.
oSecurity may wipe down the device with a chemically-sensitive wipe, which is a standard security
procedure and will not harm the device.
For international travel, use the correct universal travel power adapter. A power converter is not needed.
Only use the AC adapter that was provided with the System.
Caring for the SAVI Dual
Instruct the patient to inspect his or her device regularly.
Before using the SAVI Dual, the patient should always check to make sure the device is in good working order.
If the patient notices damage to the exterior of the device or rattling when the device is shaken, instruct the
patient to NOT USE the device and contact an eNeura representative for a replacement.
Rechargeable Battery
The rechargeable battery in the SAVI Dual is in a sealed compartment and may only be replaced by a trained
service technician. If the battery fails to recharge, please contact eNeura Customer Care to arrange for a
device exchange.
Cleaning Procedure
Instruct the patient to clean the device as described below:
eNeura recommends cleaning the SAVI Dual every three months, similar to the way one would clean a
telephone handset.It may be necessary to clean the System more often if it becomes dirty or sticky.It may
be cleaned as many times as needed.
Only the external surfaces of the System may be cleaned.Wipe dust and lint away with a dry cloth. Take care
not to drip or spray liquid directly onto your patient’s device or get liquid into any of the ports, the buttons or
the display.Dampen a soft cloth in water premixed with a mild detergent or an alcohol- based, hospital-
grade, hand-disinfecting solution.Wring out any excess liquid, and then wipe the device.Immediately dry the
device with a soft dry cloth to make sure that no liquid remains on the surface.
Do not use any spray cleaners because fluid should not enter openings. Do not use detergents or other
cleaning solutions to clean the display.
•Precaution: Disconnect the SAVI Dual from the battery charger prior to cleaning.NEVER
immerse the System.Do NOT drip or splash any liquid on the device. Contact eNeura if the
device gets wet.Do not attempt to sterilize the System.
Other manuals for SAVI
2
Table of contents
Other eNeura Personal Care Product manuals