ETION CREATE CHANNELS MEDICAL SOLUTONS CUBE 25 NIV User manual

COVID-19 setup guide
CUBE 25 NIV

I
2 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
CUBE 25 NIV
The Cube 25 is manufactured in
South Africa under license from
JFR Medical Instruments.
It
is functionally equivalent to
the
CUBE 30 and can be used for
emergency treatment during the
COVID-19 Pandemic.
This ventilator (CUBE 25 NIV)
is not registered by the
Authority and is only
authorized for emergency use
during the Covid-19
pandemic.
Decommissioning and Disposal
•
The device is designed with lifetime of five years.
•
Authorization is only provided for emergency use during the COVID-19
pandemic.
•
Etion Create intends to register a CE Mark of the CUBE 25 NIV and shall inform
customers as and when this will be available.
•
Please contact your supplier after 31 March 2021 for expanded application/
clinical indication information, instructions for disposal or product update
options.
Regulatory Status and Use Authorization
The maximum FiO
2
delivered by the Cube 25 NIV is 80%
Oxygen supplementation to the mask only (without oxygen supply via nasal prongs)
seems to be not suitable for the purpose of respiratory therapy in hypoxic patients.
WARNING

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
3
Key VentilationFeatures
1.
The CUBE 25 NIV is a device for non-invasive, non-life supporting ventilation of
spontaneously breathing patients who have a body weight of at least 13 kg and
suffer from respiratory insufficiency. It is not intended to be a replacement for life
supporting respiratory equipment.
2.
The CUBE 25 NIV ventilator is manufactured in South Africa to address the shortage of
ventilators for treating COVID-19 patients.
3.
The device has a Clinical and Patient mode. Once the device is set up by a clinician,
the day to day operation can be performed by the Patient or Care Worker.
4.
Clinical and User Manual can be found here:
https://www.parsec.co.za/downloads/#cube25
Feature
CPAP
PSV-S
PSV-ST
PCV
APCV
Range
CPAP
•
3 to 20 hPa
IPAP
•
•
•
•
5 to 25 hPa
EPAP
•
•
•
•
3 to 23 hPa
Target
Volume
•
•
•
•
0 to 2000ml
Backup rate
•
•
•
4 to 30 bpm
Inspiration Trigger
•
•
•
1 to 5 steps
Expiration Trigger
•
•
Auto, 30 to 70%
Trigger Lock
•
•
•
0,3 to 8 sec
Inspiration Time
•
•
•
0,4 to 5 sec
T
i
Min
•
•
0,3 to 5 sec
T
i
Max
•
•
0,4 to 5 sec
Inspiration slope
•
•
•
•
1 to 6 steps
Expiration Slope
•
•
•
•
1 to 6 steps
Alarms
•
•
•
•
•
Audio and
visual
FiO2 when O2 administered to
nasal prongs and breathing tube
•
•
•
•
•
0 - 80%
Intended Use

I
4 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
Device Interfaces
For further details refer to the User Manual
Device Controls
Start/Stop

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
5
Connecting the device to UPS
1.
Ensure the therapy device is turned off.
2.
Plug in the AC adaptor into the Cube 25 NIV. Clamp the cord securely to the Cube 25 NIV
enclosure.
3.
Plug the AC adaptor into the one of the clearly marked battery backed-up power sockets on
the UPS unit. Ensure that the UPS remains a minimum of 0.5 meters from the Cube 25 NIV
and patient.
NOTE
The UPS is not intended to be used to power your Cube 25, but for backup power purposes in the event of
an unexpected loss of power. The UPS must be placed at least 0.5 m away from the Cube 25 and patient.
Be certain to plug the UPS power cord directly into a wall outlet and not into a surge protector. The UPS
outlets are powered whenever the UPS is switched ON. During a power outage, the UPS outlets will be
powered for a limited time depending on the specifications of the UPS. The UPS may take up to 6 hours to
complete a full charge.
Recommended minimum specifications for an Uninterruptible Power Supply
•Uninterruptible Power Supply: 110 to 240 v
•Operation time: > 30min

I
6 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
Selecting Clinical/PatientModes
Initial setup by Physician
4.
Decide on treatmentmode
5.
Select clinical mode
6.
Define therapy parameters
7.
Select therapy set
8.
Exit clinicalmode
New Patient
9.
Disinfect CUBE 25 NIV device
10.
Replace filter, mask etc.
11.
Assemble new patient interface
-Refer to manufacturer instructions
12.
Connect to CUBE 25 NIV to test
13.
Check unobstructed flow
Treatment
14.
Place nasal prongs in patient nostrils and start
administration of oxygen at10L/min
15.
With nasal prongs in situ, place the mask on
patient
16.
Press the Start/Stop button on CUBE 25 to
start flow
17.
Check unobstructed flow (remove plastic
rectangular filter cover)
18.
Connect CUBE 25 to mask
19.
Connect second oxygen feed AFTER starting
CUBE 25
20.
Adjust flow (if required) on both oxygen feeds
to required levels
21.
Monitor patient O2 saturation, FiO2 andCO2
22.
Respond to alarms as necessary
-Audio pause will silence alarm for two minutes
23.
Turn off second oxygen supply at end of therapy
24.
Stop therapy on CUBE 25 after turning off second
oxygen feed
ClinicalGuide
UserGuide
The device starts, in Patient mode.
The setting options are limited in this mode.
Display colours are Green or Purple in Patient
mode.
To switch between Patient mode
and Clinical mode, press
Audio Pause and OK simultaneously
The display colour changes to Blue ,
The message “Clinical mode - Medical experts
only” appears.
Refer to the Clinical Guide for more information
Clinical Workflows

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
7
25.
CPAP
26.
PSV-S / Bilevel S
27.
PCV Bilevel T
28.
PSV Bilevel ST
29.
APCV
Refer to the Clinical Guide for more information
The Cube 25 NIV has been tested for the following modes:
•CPAP – Refer to Oxygen flow measurements for CPAP mode on page 9 of this document
•PSV/Bilevel ST (using a breath rate of 12 bpm) - Refer to Oxygen flow measurements for PSV/Bilevel ST
mode on page 9 of this document
The Cube 25 NIV must be setup in the Therapy Settings of the device for Tube Length of 1.8m / 22mm and the
setting for the Bacterial Filter must be turned to YES.
The unit of measure is selectable in the Therapy Settings.
Selecting Therapy Modes and Therapy Sets
CAUTION A physician must set the correct therapy.
CUBE 25 NIV Settings for COVID-19Treatment
A physician is required to select the therapy
mode and program the parameters in Clinical
mode.
Maximum two therapy sets can be
programmed.
Once the therapy set has been set up, the
therapy set can be selected by trained staff or
the Patient.

I
8 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
#
Item Part number Supplier
1
Standard Oxygen tubing with Nasal Prongs
RCNC1161
CMS
2Breathing tube system, Ø 22 mm, 1.80 m
P001Z002
Included with CUBE 25
3
Straight connector 22M - 6mm OD stem and
cap - 22F (with cap removed to ensure leak)
1963000 CMS
4Bacterial / Viral filter – in/exhalation
1690001
CMS
5
Oxygen adaptor for secondary oxygen feed
CCSC1963
CMS
6Anaesthetic mask Size 3, 4 or 5 1514 or 1515 or 1516 CMS
7
Standard Oxygen tubing for secondary oxygen feed
1174000 CMS
8Uninterruptable Power Supply (Not shown on image) UPS850LI CMS
CMS: Channels Medical Solutions
Mask Patient Circuit Setup
Mask Breathing Circuit Component List

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
9
Oxygen flow measurements for CPAP mode
CPAP
Setting
(cmH2O) 5l/min NP* 5l/min NP* +
5l/min Feed 2** 5l/min NP* +
10l/min Feed 2** 10l/min NP* +
10l/min Feed 2**
5 40.8% 67.2% 80.0% 94.2%
10 32.3% 52.1% 62.6% 80.1%
15 29.1% 43.9% 54.9% 72.2%
20 32.8% 38.6% 48.8% 66.1%
Oxygen flow measurements for PSV/Bilevel ST mode
Setting
(cmH2O)
EPAP/IPAP 5l/min Feed 2** 5l/min NP* +
5l/min Feed 2** 5l/min NP* +
10l/min Feed 2** 10l/min NP* +
10l/min Feed 2**
5/10 47.7% 58.2% 73.1% 90.2%
10/15 37.9% 47.1% 58.6% 79.2%
*NP – Oxygen feed via Nasal Prongs (Refer to item 1 in Mask Patient Circuit Setup on page 8 of this document)
**Feed 2 – Secondary Oxygen feed (Refer to item 7 in Mask Patient Circuit Setup on page 8 of this document)
NOTES:
•
This could be a bridge therapy for patients that require more than just oxygenation supplementation.
•
The oxygenation of patients should be continuously monitored.
•
Oxygen supplementation to the mask only (without oxygen supply via nasal prongs) seems to be not
suitable for the purpose of respiratory therapy in hypoxic patients.
Oxygen Enrichment Measurements
Oxygen must be administered under the supervision of a physician
Do not use if damaged
CAUTION
The maximum FiO
2
delivered by the Cube 25 NIV is 80%
Oxygen supplementation to the mask only (without oxygen supply via nasal prongs)
seems to be not suitable for the purpose of respiratory therapy in hypoxic patients.
WARNING

I
10 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
Audible and visible alarms indicate various fault conditions.
High priority
The result of a high-priority alarm can be the death or irreversible injury to
the patient. This is shown in the device display by three red triangles.
An acoustic signal also sounds
Moderate priority
The result of a moderate-priority alarm can be reversible injury to the
patient. It is shown in the device display by two yellow triangles.
An acoustic signal also sounds
Low priority
The result of a low-priority alarm can be minor, reversible injuries to the
patient or slight damage to the device. It is shown in the device display by a
turquoise triangle. An acoustic signal also sounds
Acoustic signals
In addition to the visual signals in the device display the device also issues an acoustic signal.
This consists of a sequence of tones that vary depending on the nature of the alarm and
priority.The sequence of tones is indicated by the letters forming each tone pitch (c, a, f, e).
The C is an octave above middle C.
Display Priority
Acoustic
Cause
Trigger
Resolution
Circuit
disconnection
High
c a f - a f
Fall in pressure during
exhalation.
Tube system probably detached.
10 sec
Check tube
connections.
Mains failure Moderate
2 min.
Beep
The device no longer has
power supply.
Direct
Check the power and
UPS connections.
Tube blocked
Moderate
c a f
Obstruction in the tube system.
10 sec
Check for blockages.
Remove and restart
therapy.
System error Moderate
C c c
There is an internal error or
EM interference. Direct Refer to User Manual for
more detail.
High pressure
Moderate
c a f
Therapy pressure exceeds set
limit value.
10 sec
Stop the therapy. Refer
to User Manual for more
detail.
Leakage
Low
e c
High system flow (leakage)
detected. The respiratory mask
has possibly slipped.
10 sec
Check the fit of the
respiratory mask.
Mon vented mask
Moderate
c a f
A respiratory mask is being
used without an air outlet,
or the air outlet is blocked.
10 sec
Ensure patient is using
mask with air outlet.
Check that air outlet is not
blocked.
For further details refer to the User Manual
Alarms (1/3)
Alarms (2/3)

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
11
Display Priority
Acoustic
Cause
Trigger Resolution
Low minute volume
Moderate
c a f The respiratory minute volume
is lower than the set limit value. 10 sec
The patient’s condition
should be checked.
High respiratory rate
Low
e c
The respiratory rate exceeds
the set limit value. 5 sec
The patient’s condition
should be checked.
Low respiratory
rate
Moderate
c a f The respiratory rate is below
the set limit value. 5 sec
The patient’s
condition
should be checked.
Target volume not
reached
Moderate
c a f
The target volume is not
reached despite the maximum
indicated inspiration pressure.
Immediately
The patient’s condition
should be checked.
Target minute
volume not reached
Moderate
c a f
The target minute volume
is not reached despite the
maximum indicated inspiration
pressure.
Immediately
The patient’s condition
should be checked.
Press the Audio Pause button to silence the sound for two
minutes while addressing theissue
For further details refer to the User Manual
Alarms (3/3)

12 Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
Components Interval Activity
Breathing device
CUBE 25 NIV
When patient changed
Disinfect the device surfaces
Power cable
When patient changed
Disinfect the power cable
Coarse filter At the latest after 1 500 operating hours or
when changing patients
Replace coarse filter,
earlier if damaged
Fine filter Every 1 000 operating hours or when dirty Replace fine filter
Transport bag
When patient changed
Replace transport bag
Blower Every 20 000 operating hours
Have the device serviced at
supplier for safety check and
blower replacement.
1)Appropriate PPE must be worn when the device is decontaminated (K95 mask, face shield, gloves and
coveralls.
2) Perform cleaning in a well-ventilated area.
3) Remove coarse and fine filter.
4)Use a 3% Ready to Use RBT - Protectus Viridis solution. (Follow manufacturer instructions.)
5) Spray the outside of the device with RBT - Protectus Viridis solution.
6) Use a clean lint free cloth and wipe all obvious dirt from the device.
7)Spray the device again and wait for 5 minutes (disinfectant contact time).
8)Connect the Cube 25 NIV to ResComf XD100 (or equivalent) for a period of 30 minutes. (Follow manufacturer
instructions.)
9) Wipe off any excess disinfectant with a clean lint free cloth.
10) Replace coarse and fine filter before using it on the next patient.
CUBE 25 NIV Service, Maintenance and Repair
If the device is used without Bacterial/Viral filter or contamination is suspected,
contamination disinfection protocol shall be followed. Contact supplier for instructions.
WARNING Never use if damaged.
CUBE 25 NIV Cleaning Instructions

Doc. Ref.: ETION-MED-MDF-01-06-08 (Rev 02)
I
13
ETION Create (Pty) Ltd, (“the Manufacturer”) warrants that the Cube 25 NIV (“the Goods”), exclusive of expendable parts and other
accessories, shall be free from defects in material and workmanship for a period of 12 (twelve) months from the original date of delivery, and
shall conform to its specification at the time of delivery.
The Manufacturer appoints authorised resellers (“Suppliers”) to resell and distribute the Goods on its behalf. All warranty communications
must be directed at the Supplier from whom your Goods have been purchased.
The Manufacturer shall within the warranty period repair or replace, at its sole discretion, any item which is proven to the Manufacturer’s
reasonable satisfaction to be defective, provided that:
The Customer has given written notice to the Supplier within 30 (thirty) days of the discovery of a defect;
The Goods have not been modified in any way;
Such defects did not occur due to inadequate storage, incorrect or misuse, improper handling, improper maintenance, tampering or
negligence by any person;
The Goods were used under normal operating conditions and in accordance with the Manufacturer’s written instructions.
In the event of the warranty becoming void as a result of an occurrence specified in paragraph 3a to 3d above, the Manufacturer will provide
the Customer with a written notice of its reason for rejecting the warranty claim.
The Customer shall, in accordance with the Supplier’s written instructions, deliver the defective item(s) at the Customer’s expense, at a
location designated by the Supplier, in which event the said item(s) will be repaired or replaced according to the Manufacturer’s standard
practices and procedures.
Any item which is replaced will become the property of the Manufacturer. Upon completion of the repair/replacement, the Supplier shall
deliver the repaired or replaced item to the Customer at the original delivery address.
The provisions of the warranty shall apply to a repaired/replacement item for the remainder of the warranty period.
This warranty is exclusive and in lieu of all other warranties, obligations and/or liabilities, express or implied, whether arising in contract,
delict, law, including negligence, or otherwise, and the Manufacturer’s/Supplier’s liability is restricted to the repair or replacement of the
defective item. In no event shall the Manufacturer or the Supplier be liable for incidental, special and/or consequential damages.
Warranty
Table of contents
Popular Medical Equipment manuals by other brands

AREQUIPMENT
AREQUIPMENT Monobloc 4SW instruction manual
Fresenius Medical Care
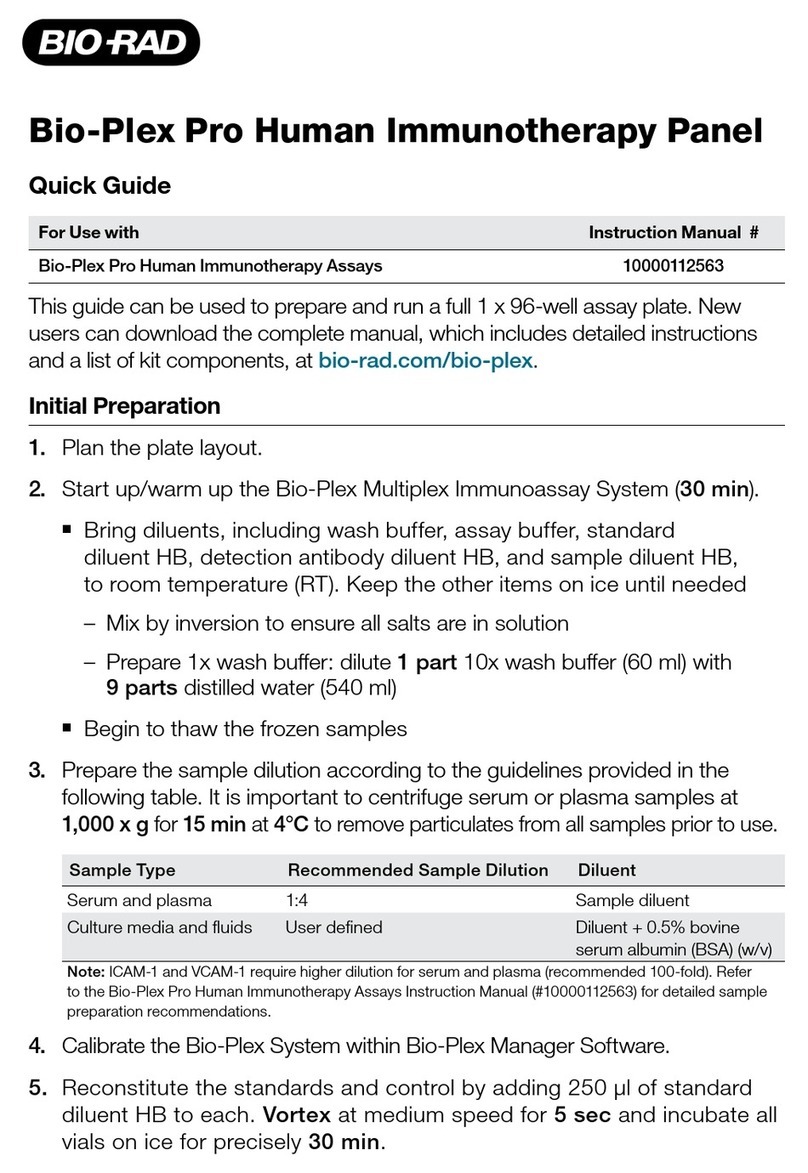
Fresenius Medical Care Dialysis Water Distribution Loop Instructions for use
Liko
Liko MultiStrap Instruction guide

Abbott
Abbott Amplatzer TorqVue Instructions for use

Epocal
Epocal Epoc Host quick start guide

Stryker
Stryker 4701 Operation manual

laerdal
laerdal SimPad quick start guide
Otto Bock
Otto Bock AxonSkin Silicone 8S511 Series Instructions for use

XFT Medical
XFT Medical XFT-120G Directions for use
Fresenius Medical Care
Fresenius Medical Care multiFiltrate Instructions for use

Medstrom
Medstrom MMO 3500 user manual
Planmeca
Planmeca ProMax 3D Plus manual