Teleflex LMA Supreme User manual
Page1of4
EN–English
InstructionsForUse–
LMASupreme™
CAUTION:Federal(USA)lawrestrictsthis
devicetosalebyorontheorderofaphysician.
WARNING:LMASupreme™issuppliedsterilefor
singleuse
,
shouldbeusedstraightfromthepack
andshouldbediscardedafteruse.Itmustnotbere‐
used.Reusemaycausecrossinfectionandreduce
productreliabilityandfunctionality.
WARNING:Re‐processingofLMASupreme™
intendedforsingleuseonlymayresultindegraded
performanceorlossoffunctionality.Re‐useof
singleuseonlyproductsmayresultinexposureto
viral,bacterial,fungal,orprionicpathogens.
Validatedcleaningandsterilisationmethodsand
instructionsforreprocessingtooriginal
specificationsarenotavailableforthisproduct.
LMASupreme™isnotdesignedtobecleaned,
disinfected,orre‐sterilised.
1.DEVICEDESCRIPTION:
TheLMASupreme™isaninnovative,second
generation,singleusesupraglotticairway
managementdevice.
TheLMASupreme™providesaccesstoand
functionalseparationoftherespiratoryanddigestive
tracts.Theanatomicallyshapedairwaytubeis
ellipticalincrosssectionandendsdistallyatthe
laryngealmask.Theinflatablecuffisdesignedto
conformtothecontoursofthehypopharynx,with
thebowlandthemaskfacingthelaryngealopening‐
theFirstSeal™.
TheLMASupreme™alsocontainsadraintubewhich
emergesasaseparateportproximallyandcontinues
distallyalongtheanteriorsurfaceofthecuffbowl,
passingthroughthedistalendofthecuffto
communicatedistallywiththeupperoesophageal
sphincter‐theSecondSeal™.
Thedraintubemaybeusedforthepassageofawell
lubricatedgastrictubetothestomach,offeringeasy
accessforevacuationofgastriccontents.Thedrain
tubehasanadditionalandimportantfunction–it
maybeusedasamonitorofcorrectpositioningof
theLMASupreme™followinginsertionandthenfor
continuousmonitoringofmaskdisplacementduring
use.
TheLMASupreme™provideseasyinsertionwithout
theneedfordigitalorintroducertoolguidanceand
enoughflexibilitytopermitthedevicetoremainin
placeifthepatient’sheadismovedinanydirection.
Thetwolateralgroovesintheairwaytubeare
designedtopreventtheairwaytubekinkingwhen
flexed.Abuilt‐inbite‐blockreducesthepotentialfor
tubedamageandobstructionbypatientbiting.
TheLMASupreme™hasanewfixationsystemwhich
preventsproximaldisplacement.Ifcorrectlyused,
thisenhancethesealofthedistalendaroundthe
upperoesophagealsphincter‐SecondSeal™thereby
isolatingtherespiratorytractfromthedigestivetract,
soreducingthedangerofaccidentalaspiration.
Attachedtothemaskisacuffinflationline
terminatinginapilotballoonandone‐waycheck
valveformaskinflationanddeflation.
TheLMASupreme™ismadeprimarilyofmedical
gradepolyvinylchloride(PVC)andissuppliedsterile
forsingleuseonly.Itisterminallysterilizedby
EthyleneOxidegas.
Allcomponentsarenotmadewithnaturalrubber
latex.
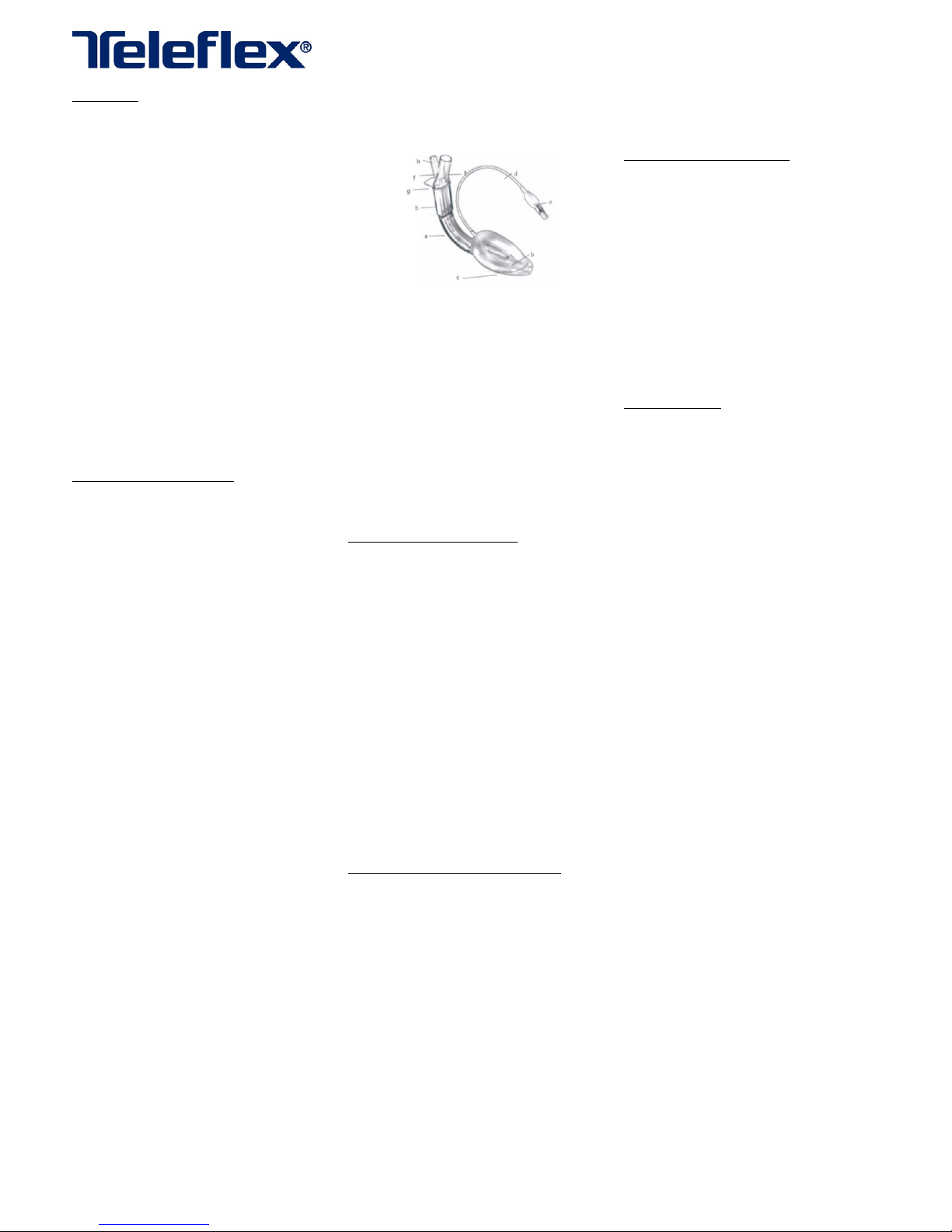
Figure1:LMASupreme™components
LMASupreme™components(Figure1):
(a)Anatomically‐shapedairwaytube
(b)Aseparatedraintubehasbeenincorporated
(c)Inflatablecuffwithinterlockingproximaland
distalsegments
(d)Cuffinflationline
(e)Pilotballoon
(f)Arigidmouldedproximalcomponentwhichforms
separateairwayanddraintubeports
(g)Fixationtab
(h)Integralbite‐block
Thedeviceisonlyforusebymedicalprofessionals
trainedinairwaymanagement.
2.INDICATIONSFORUSE:
TheLMASupreme™isindicatedforuseinachieving
andmaintainingcontrolofairwayduringroutineand
emergencyanaestheticproceduresinfastedpatients
usingeitherspontaneousorpositivepressure
ventilation.
Itisalsoindicatedforuseastherescueairwaydevice
inCPRprocedures,inwhichtheLMAProSeal™,LMA
Classic™ortheLMAUnique™havetraditionallybeen
used.TheLMASupreme™isalsoindicatedasa
“rescueairwaydevice”inknownorunexpected
difficultairwaysituations.TheLMASupreme™may
beusedtoestablishanimmediateclearairway
duringresuscitationintheprofoundlyunconscious
patientwithabsentglossopharyngealandlaryngeal
reflexeswhomayneedartificialventilation.
Itmayalsobeusedtosecureanimmediateairway
whentrachealintubationisprecludedbylackof
availableexpertiseorequipment,orwhenattempts
attrachealintubationhavefailed.
3.RISK‐BENEFITINFORMATION:
ThebenefitsofestablishingventilationwiththeLMA
Supreme™mustbeweighedagainstthepotential
riskofaspirationinsomesituationsincluding:
symptomaticoruntreatedgastro‐esophagealreflux,
pregnancyover14weeks,multipleormassiveinjury,
conditionsassociatedwithdelayedgastricemptying,
suchastheuseofopiatemedicationinpatientswith
acuteinjuryorperitonealinfectionsorinflammatory
processes.
Whenusedintheprofoundlyunresponsivepatientin
needofresuscitationorinadifficultairwaypatient
onanemergencypathway(i.e.“cannotintubate,
cannotventilate”)theLMASupreme™isthe
preferentialairway“rescue”devicetoensure
oxygenation.Theriskofregurgitationandaspiration
isminimizedastheLMASupreme™offerseasy
accesstoliquidgastriccontents.However,the
ultimatechoiceofthedefiniteairway“rescue”
deviceremainswiththeairwaymanager.
Inpatientswithsevereoropharyngealtrauma,the
deviceshouldonlybeusedwhenotherattemptsto
establishanairwayhavefailed
.
4.CONTRAINDICATIONS:
‐ Patientswhohavehadradiotherapytotheneck
involvingthehypopharynx(riskoftrauma,failureto
sealeffectively).
‐Patientswithinadequatemouthopeningtopermit
insertion.
‐Patientspresentingforemergencysurgerywhoare
atriskofmassivereflux,suchasacuteintestinal
obstructionorileus,orpatientshavingbeeninjured
shortlyafteringestingasubstantialmeal(butsee
aboveunderIndicationforUse).
‐ Patientsrequiringheadornecksurgerywherethe
surgeoncannotgainadequateaccessduetothe
presenceofthedevice.
‐Responsivepatientswithanintactgagreflex.
‐Patientswhohaveingestedcausticsubstances.
5.WARNINGS:
5.1Inspiteofencouragingcasereports,itisnot
currentlyknownwhethertheLMASupreme™
alwaysaffordsprotectionfromaspirationeven
whencorrectlyfixedinplace.
5.2Thepresenceofagastrictubedoesnotruleout
thepossibilityofaspirationifthedeviceisnot
correctlylocatedandfixedinplace.
5.3TheLMASupreme™maybeineffectiveforusein
patientswithdecreasedpulmonarycompliancedue
tofixedobstructiveairwaysdiseasebecauseairway
positivepressurerequirementmayexceedseal
pressure.
5.4Donotattempttopassagastrictubeintothe
stomachviathedraintubeinthepresenceof
knownorsuspectedoesophagealpathology.
5.5Thereisatheoreticalriskofcausingoedemaor
haematomaifsuctionisapplieddirectlytotheend
ofthedraintube.
5.6Toavoidtrauma,excessiveforceshouldnotbe
usedatanytimewhenusingthedevices.Excessive
forcemustbeavoidedatalltimes.
5.7ThisdevicecontainsDi(2‐ethylhexyl)phthalate
(DEHP).Theresultsofcertainanimalexperiments
haveshownphthalatestobepotentiallytoxicto
reproduction.Proceedingfromthepresentstateof
scientificknowledge,risksformalepremature
infantscannotbeexcludedinthecaseoflong‐term
exposureorapplication.Medicalproducts
containingphthalatesshouldbeusedonly
temporarilywithpregnantwomen,nursingmothers,
babiesandinfants.
5.8DonotusetheLMASupreme™ifthedeviceis
damagedortheunitpackagingisdamagedor
opened.
5.9Neverover‐inflatethecuffofthedeviceover60
cmH
2
O.Excessiveintra‐cuffpressurecanresultin
malpositionandpharyngo‐laryngealmorbidity,
includingsorethroat,dysphagiaandnerveinjury.
5.10Donotimmerseorsoakthedeviceinliquid
priortouse.
5.11Itismostimportantthatpre‐usechecksare
carriedoutonthedevicepriortouse,inorderto
establishwhetheritissafeforuse.Failureofany
onetestindicatesthedeviceshouldnotbeused.
5.12.Whenapplyinglubricantavoidblockageofthe
airwayaperturewiththelubricant.
5.13Awater‐solublelubricant,suchasK‐YJelly®,
shouldbeused.Donotusesilicone‐based
lubricantsastheydegradeLMASupreme™
components.LubricantscontainingLidocaineare
notrecommendedforusewiththedevice.
Lidocainecandelaythereturnofthepatient’s
protectivereflexesexpectedpriortoremovalofthe
Page2of4
device,maypossiblyprovokeanallergicreaction,or
mayaffectthesurroundingstructures,includingthe
vocalcords.
5.14Diffusionofnitrousoxide,oxygen,orairmay
increaseordecreasecuffvolumeandpressure.In
ordertoensurethatcuffpressuresdonotbecome
excessive,cuffpressureshouldbemeasured
regularlyduringacasewithacuffpressuremonitor.
5.15Whenusingthedeviceinspecialenvironmental
conditions,suchasenrichedoxygen,ensurethatall
necessarypreparationandprecautionshavebeen
taken,especiallywithregardtofirehazardsand
prevention.Thedevicemaybeflammableinthe
presenceoflasersandelectrocauteryequipment.
5.16Refertosection18forMRIinformationpriorto
usingthedevicesinMRIenvironment.
6.CAUTIONS:
6.1Onlyusewiththerecommendedmanoeuvres
describedintheinstructionsforuse.
6.2Ifairwayproblemspersistorventilationis
inadequate,theLMASupreme™shouldberemoved
andanairwayestablishedbysomeothermeans.
6.3Carefulhandlingisessential.TheLMASupreme™
ismadeofmedical‐gradePVCwhichcanbetornor
perforated.Avoidcontactwithsharporpointed
objectsatalltimes.Donotinsertthedeviceunless
thecuffisfullydeflatedasdescribedinthe
instructionsforinsertion.
6.4Glovesshouldbewornduringpreparationand
insertiontominimizecontaminationoftheairway.
6.5Storethedeviceinadarkcoolenvironment,
avoidingdirectsunlightorextremesof
temperature.
6.6Useddeviceshallfollowahandlingand
eliminationprocessforbio‐hazardproducts,in
accordancewithalllocalandnationalregulations.
6.7Onlyuseasyringewithstandardluertapertipfor
inflation/deflationofthecuff.
6.8.Laryngealspasmmayoccurifthepatient
becomestoolightlyanaesthetizedduringsurgical
stimulationorifbronchialsecretionsirritatethe
vocalcordsduringemergencefromanaesthesia.If
laryngealspasmoccurs,treatthecause.Only
removethedevicewhenairwayprotectivereflexes
arefullycompetent.
6.9.Donotpulloruseundueforcewhenhandling
theinflationlineortrytoremovethedevicefrom
patientbytheinflationtubeasitmaydetachfrom
thecuffspigot.
6.10Ensureallremovabledentureworkisremoved
beforeinsertingthedevice.
6.11Anunreliableorobstructedairwaymayresultin
caseswherethedevicehasbeenincorrectly
inserted.
7.ADVERSEEFFECTS:
Therearereportedadversereactionsassociatedwith
theuseoflaryngealmaskairways.Standard
textbooksandpublishedliteratureshouldbe
consultedforspecificinformation.
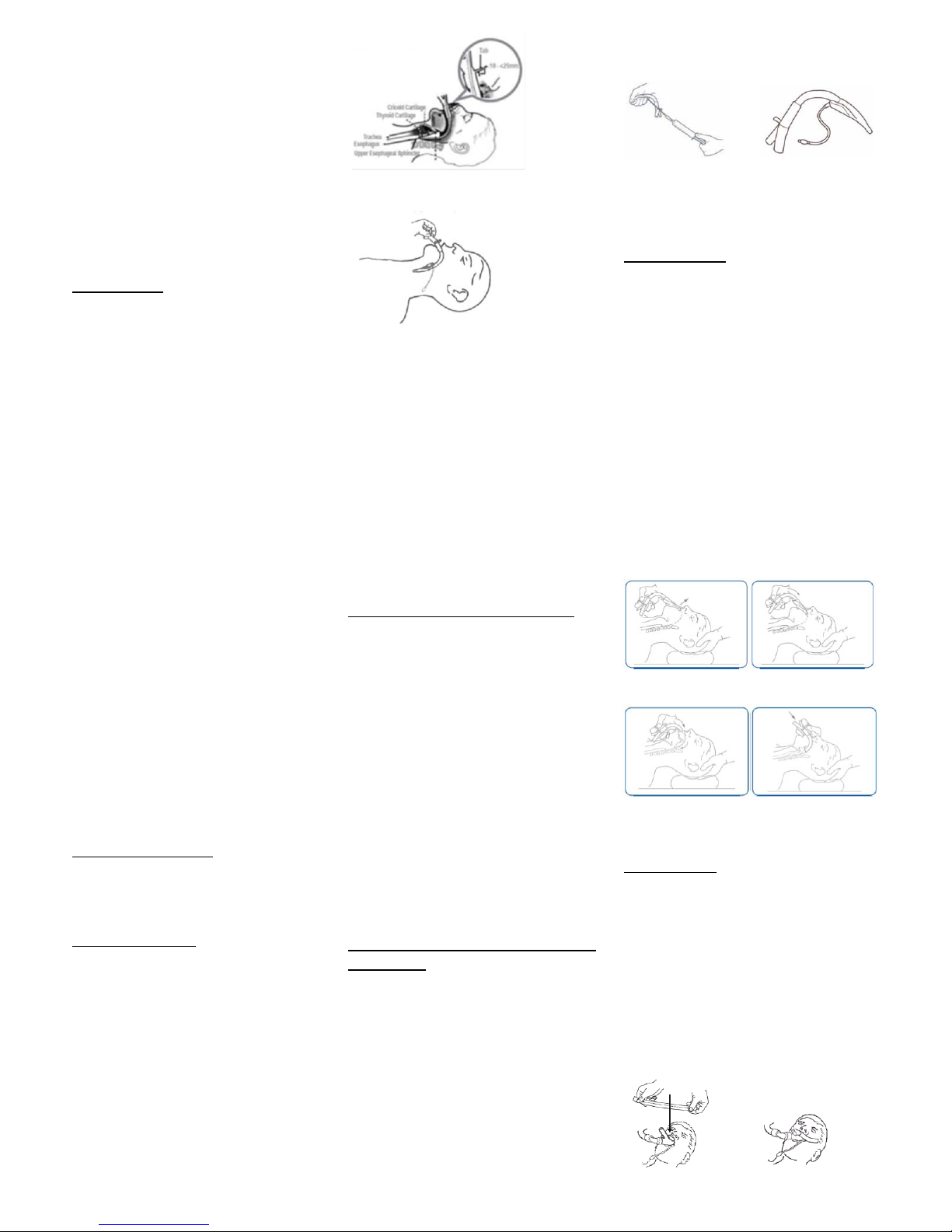
8.SIZESELECTION:
Fornormaladults,usethesize4deviceasafirst
choice.Afterinserting,fixingthedeviceinplace,and
theninflatingtotherecommendedpressure,there
shouldbeaminimumofonecmgapbetweenthe
fixationtabandthepatient’supperlip.Ifthetabis
pressingontheliporveryneartoit,thisindicates
thedeviceistoosmallforthepatientandthesize5
shouldbeusedinsteadtoavoidtheriskof(a)poor
sealagainsttheoesophagusand(b)possible
pressuretraumatothelip.Ifthefixationtabismore
than2.5cmfromtheupperlipafterfixation,itmay
beadvisabletousethesize3device.Thedecisionto
changetoasmallerdevicewilldependonthequality
ofairway,devicestabilityandsealpressureachieved.
.
Figure2:LMASupreme™sizing
Figure3:LMASupreme™sizing(method2)
Thesizingmethoddescribedaboverequiresthatall
threeadultsizesoftheLMASupreme™shouldbe
availabletohandbeforeinducinganaesthesia.
Foradultpatientswhoareeithersmallerorlarger
thannormal,itisoftenpossibletoobtainagood
resultusingthesize4device,providedthequantity
ofairusedtoinflatethecuffisalwaysbasedon
achieving60cmH₂Ointracuffpressure.Insmaller
patientsthispressureisachievedwitharelatively
smallvolumeofair,whilelargerpatientswillrequire
largervolumes.However,whenindoubt,an
approximateestimateofsuitablesizingcanbemade
byholdingeachdeviceagainstthesideofthe
patient’sfaceinthepositioncorrespondingtothat
showninFigure3.
9.PRE‐USEPERFORMANCETESTS:
Thefollowinginspectionsandtestsmustbe
conductedbeforeuseofthedevice.The
performancetestsshouldbeconductedinanarea
andinamannerconsistentwithacceptedmedical
practicethatminimisecontaminationoftheLMA
Supreme™beforeinsertion.
Warning:Donotusethedeviceshoulditfailsany
oneoftheinspectionsortests.
•ExaminethesurfaceoftheLMASupreme™and
draintubefordamage,includingcuts,tears,
scratchesorkinks.
•Examinetheinterioroftheairwaytubeanddrain
tubetoensuretheyarefreefromblockages
kinkingofthedraintubewithintheairwaytube
orlooseparticles.Anyparticlespresentinthe
tubesshouldberemoved.Donotusetheairway
iftheblockageorparticlecannotberemoved.
•Deflatethecuffcompletely.Oncedeflated,check
thecuffforspontaneousinflation.Donotuse
theairwayifthecuffspontaneouslyinflates.
10.DEFLATINGTHEDEVICEPRIORTO
INSERTION:
‐Afterfirmlyconnectingasyringeofatleast50mlto
theinflationport,holdthesyringeandtheLMA
Supreme™exactlyasshowninFigure4.Movethe
connectedsyringeawayfromthedeviceuntilthe
inflationlineisslightlystretchedasshown.
Compressthedistalendofthedeviceinbetween
theindexfingerandthumbwhilewithdrawingair
untilavacuumhasbeenobtained.
‐ Whiledeflating,holdthedevicesothatthedistal
endiscurledslightlyanteriorlyasshowninFigure4
‐ Deflatethedeviceuntilthetensioninthesyringe
indicatesavacuumhasbeencreatedinthemask.
Keepthesyringeundertensionwhilstrapidly
disconnectingitfromtheinflationport.Thiswill
ensurethemaskremainscorrectlydeflated,as
showninFig5.
Figure4:LMASupreme™ Figure5:Afterachieving
deflation
wedgeshapecuffduring
deflation,disconnectthe
syringefromtheinflation
line
11.INSERTION:
Caution:Thepatencyoftheairwayshouldbe
reconfirmedafteranychangeinthepatient’shead
andneckposition.
‐ Lubricatetheposteriorsurfaceofthemaskand
airwaytubejustpriortoinsertion.
‐Standbehindorbesidepatient’shead.
‐ Placetheheadintheneutralorslight“sniffing”
position(Sniffing=extensionofhead+flexionof
neck).
‐HoldthedeviceexactlyasshowninFigure6.
‐ Pressthedistaltipagainsttheinneraspectofthe
upperteethorgums.
‐ Slideinwardsusingaslightlydiagonalapproach
(directthetipawayfromthemid‐line).
‐ Continuetoslideinwardsrotatingthehandina
circularmotionsothatthedevicefollowsthe
curvaturebehindthetongue.
‐Resistanceshouldbefeltwhenthedistalendofthe
devicemeetstheupperoesophagealsphincter.The
deviceisnowfullyinserted.
Figure6:PressthetipoftheFigure7:Pressthecufffurther
maskagainstthehardpalate.intothemouth,maintaining
pressureagainstthepalate.
Figure8:SwingthedeviceinwardFigure9:Advancethedevice
withacircularmotion,pressingintothehypopharynxuntil
againstthecontoursofthehardresistanceisfelt.
andthesoftpalate.
12.FIXATION:
SecuretheLMASupreme™topatient’sfaceusing
adhesivetapeasfollows:
‐Useapieceofadhesivetape30‐40cmlong,holding
ithorizontallybybothends
‐ Presstheadhesivetapetransverselyacrossthe
fixationtab,continuingtopressdownwardssothat
theendsofthetapeadheretoeachofthepatient’s
cheeksandthedeviceitselfisgentlypressed
inwardsbythetape
‐Donotrotatethetapearoundtheproximalendof
thedevice
‐ DonotuseaGuedelairway;thedevicehasan
integralbiteblock

Page3of4
Figure10a
Figure10b
Figure10:Fixthedeviceinplaceusingadhesivetape.
Stretchedtheadhesivetapeverticallydownwards(See
Figure10a)ensurethatthemiddleofthetapeispressed
verticallydownwardsoverthetabasshowninFigure10b.
13.INFLATION:
Inflatethecuffwithairuntilrelevantintra‐cuff
pressureisreached.Therecommendedintra‐cuff
pressureshouldneverexceed60cmH₂O.Ifno
manometerisbyhand,inflatewithjustenoughairto
achieveasealsufficienttopermitventilationwithout
leaks.
Air‐
way
Size
Patient
Weight
(kg)
Max
Size
OG
Tube
Recommended
Maximum
Inflation
Volume
Optimum
Intra‐Cuff
Pressure
1<56Fr5ml
60cm
H₂O
1.55‐106Fr8ml
210‐2010Fr12ml
2.520‐3010fr20ml
330‐5014Fr30ml
450‐7014Fr45ml
570‐10014Fr45ml
Table1:LMASupreme™selectionguide
14.CORRECTPOSITION:
Correctplacementshouldproducealeak‐freeseal
againsttheglottiswiththemasktipattheupper
oesophagealsphincter.Theintegralbiteblockshould
liebetweentheteeth.
Tofacilitatediagnosisofcorrectmaskplacement,
placeasmallbolus(1‐2ml)ofsuitablyviscouswater
solublelubricantintheproximalendofthedrain
tube.Inaproperlyplacedmask,thereshouldbea
slightup‐downmeniscusmovementofthelubricant
followingtheapplicationandreleaseofgentle
pressureinthesuprasternalnotch.Thisindicates
thatthedistalendofthedraintubeiscorrectly
placedsothatitsealsaroundtheupperoesophageal
sphincter(the‘suprasternalnotchtest’).Asimilar
movementmayalsobeseenwhengentlemanual
positivepressureisappliedtotheairwaythroughthe
device.
15.GASTRICDRAINAGE:
Thedraintubefacilitateschannelingoffluidsand
gasesemergingfromthestomach.Tofacilitate
gastricdrainage,agastrictubemaybepassed
throughthedraintubeintothestomachatanytime
duringtheanaestheticprocedure.RefertoTable1
formaximumgastrictubesizes.Thegastrictube
shouldbewelllubricatedandpassedslowlyand
carefully.Suctionshouldnotbeperformeduntilthe
gastrictubehasreachedthestomach.Suctionshould
notbeapplieddirectlytotheendofthedraintube,
asthismaycausethedraintubetocollapseand
mighttheoreticallycauseinjurytotheupper
oesophagealsphincter.
16.ANAESTHESIAMAINTENANCE:
TheLMASupreme™iswelltoleratedin
spontaneouslybreathingpatientswhenusedwith
volatileagentsorintravenousanaesthesia,provided
anaesthesiaisadequatetomatchthelevelofsurgical
stimulusandthecuffisnotover‐inflated.
DuringPositivePressureVentilation(PPV)usingthe
LMASupreme™tidalvolumesshouldnotexceed
8ml/kgandpeakinspiratorypressuresshouldbe
keptbelowthemaximumairwaysealpressure.
IfleaksoccurduringPPV,thismaybeduetolight
anaesthesiacausingadegreeofglottisclosure,
severereductioninlungcompliancerelatedtothe
procedureorpatientfactorsordisplacementor
migrationofthecuffbyheadturningortractioninan
inadequatelyfixedmask.
17.RECOVERY:
Removalshouldalwaysbecarriedoutbytrained
personnel.Althoughthedevicemaynotberemoved
intheoperatingtheatre,itslowinvasivitymakesita
gooddevicetomaintaintheairwayduringrecovery
inthePostAnaestheticCareUnit(PACU)provided
staffareappropriatelytrainedandequipped.
Becauserecoveryinvolvesincreaseinpharyngeal
tone,itmakessensetoreducethevolumeofairin
thecuffbeforesendingthepatienttothePACU;
however,thecuffmustneverbefullydeflatedatthis
point.
Fullydeflatethecuffandsimultaneouslyremovethe
deviceONLYwhenthepatientcanopenthemouth
oncommand.IfthecuffisFULLYdeflatedbeforethe
returnofeffectiveswallowingandcoughreflexes,
secretionsintheupperpharynxmayenterthe
larynx,provokingcoughingorlaryngealspasm.
Patientmonitoringshouldcontinuethroughoutthe
recoverystage.Whereappropriate,oxygenmaybe
continuouslyadministeredthroughtheanaesthetic
circuitorviaaT‐pieceattachedtotheproximalend
oftheairwaydevice.
18.USEWITHMAGNETICRESONANCE
IMAGING(MRI):
TheLMASupreme™isMRConditional.Non‐clinical
testingdemonstratedthattheLMASupreme™isMR
Conditional.Apatientwiththisdevicecanbe
scannedsafely,immediatelyafterplacementunder
thefollowingconditions:
BeforethepatiententerstheMRIsystemroom,
theairwaymustbefixedproperlyinplacewith
adhesivetape,clothtapeorotherappropriate
meanstopreventmovementordislodgement.
Staticmagneticfieldof3‐Teslaorless.
Maximumspatialgradientmagneticfieldof
720gauss/cm(7.2T/m)orless.
MaximumMRsystemreported,wholebody
averagedspecificabsorptionrate(SAR)of4‐
W/kg(FirstLevelControlledOperatingModeof
operationfortheMRIsystem)for15min.of
scanning(perpulsesequence).
MRI‐RelatedHeating
Underthescanconditionsdefinedabove,theLMA
Supreme™isexpectedtoproduceamaximum
temperatureriseof2.2Cafter15minutesof
continuousscanning.
ArtifactInformation
Themaximumartifactsizeasseenonagradientecho
pulsesequenceanda3‐TeslaMRIsystemextends
approximately20‐mmrelativetothesizeandshape
oftheLMASupreme™,Size5.
19.SYMBOLSDEFINITION:
Manufacturer
ConsultIFUonthiswebsite:
www.LMACO.com
Airinflationvolume
Patientweight
ReadInstructionsbeforeuse
Notmadewithnaturalrubber
latex
Fragile,handlewithcare
Keepawayfromsunlight
Keepdry
Thiswayup
ProductCode
LotNumber
CEMark
DonotRe‐use
DonotRe‐sterilise
ContainsorPresenceof
Phthalates:
Bis(2‐ethylhexyl)phthalate
(DEHP)
SterilisedbyEthyleneOxide
UseBy
Donotuseifpackageis
damaged
MRConditional

Page4of4
Copyright©2015TeleflexIncorporated
Allrightsreserved.Nopartofthispublicationmaybe
reproduced,storedinaretrievalsystemor
transmittedinanyformorbyanymeanselectrical,
mechanical,photocopying,recordingorotherwise,
withoutthepriorpermissionofthepublisher.
LMA,LMABetterbyDesignandLMASupremeare
trademarksorregisteredtrademarksofTeleflex
Incorporatedoritsaffiliates.TheLMASupreme™is
protectedbyaseriesofgrantedandpendingpatents.
Theinformationgiveninthisdocumentiscorrectat
thetimeofgoingtopress.Themanufacturer
reservestherighttoimproveormodifytheproducts
withoutpriornotification.
Consulttheinstructionsonindications,
contraindications,warningsandprecautions,or
informationonwhichLMA™airwaysarebestsuited
fordifferentclinicalapplications.
Manufacturer’sWarranty:
TheLMASupreme™isdesignedforasingleuseand
warrantedagainstmanufacturingdefectsatthetime
ofdelivery.
Warrantyisapplicableonlyifpurchasedfroman
authorizeddistributor.TELEFLEXINCORPORATED
DISCLAIMSALLOTHERWARRANTIES,WHETHER
EXPRESSORIMPLIED,INCLUDING,WITHOUT
LIMITATION,THEWARRANTIESOF
MERCHANTABILITYORFITNESSFORAPARTICULAR
PURPOSE.
TeleflexMedical
IDABusinessandTechnologyPark
DublinRoad,Athlone
Co.Westmeath,Ireland
ContactInformationinUSA:
TeleflexMedical
2917WeckDrive,ResearchTrianglePark
NC27709USA
International:(919)544‐8000
USA:(866)246‐6990
www.LMACO.com
Issue:PAJ‐2100‐002RevBUK
Other Teleflex Medical Equipment manuals

Teleflex
Teleflex Arrow EZ-IO User manual
Teleflex
Teleflex WECK EFx Shield User manual
Teleflex
Teleflex LMA Unique Cuff Pilot User manual

Teleflex
Teleflex LMA Supreme User manual

Teleflex
Teleflex Arrow PICC User manual

Teleflex
Teleflex LMA Unique PreCurved User manual

Teleflex
Teleflex ARROW GlideThru User manual

Teleflex
Teleflex Arrow EZ-IO User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











