GB
10
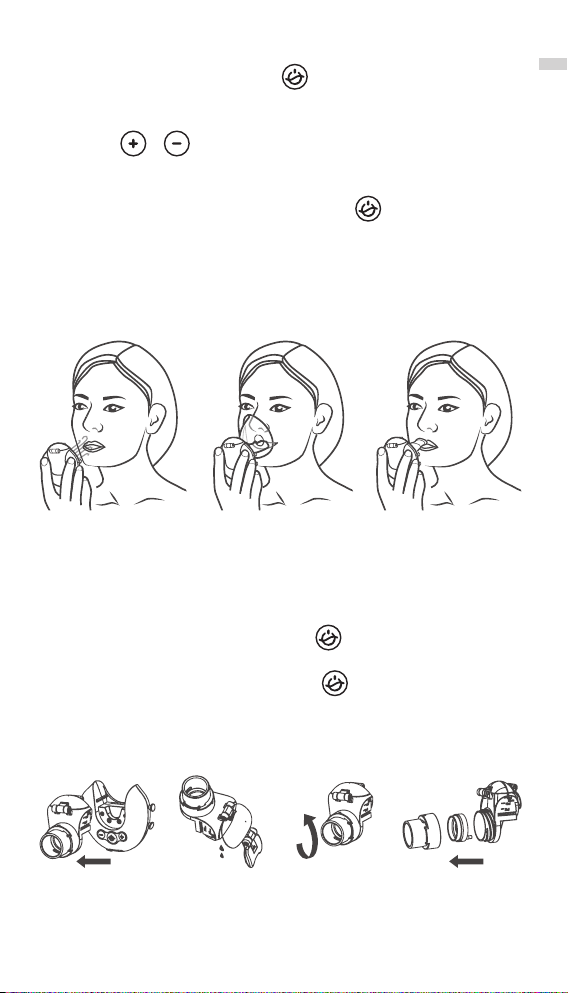
8) Use purified water to clean the medication cup, spray assembly, lid, thread
assemblies and accessories, and then follow the recommended method of
disinfection.
Note: 1) Host nebulizer to keep the liquid and nebulization mesh disc in full con-
tact. Slight swing does not aect the use but do not lean back the nebulization.
2) Receiving treatment according to the doctors’ recommendation. Keep quiet
and relaxed in the course of treatment.
3) Liquid will be coagulated around the spray assembly and mesh disc, which
will aect the nebulization eect after nebulization. It should stop nebuliza-
tion, remove the mouthpiece and other accessories. Then use a clean
medical gauze to wipe the residue. Do not touch the mesh disc center spray
area to prevent damage to the mesh disc.
4) Essential oils, cough syrups, gargling solutions and drops to be used as a rub
or in a steam bath are wholly unsuitable for inhalation using a nebuliser. These
additives are often viscous and can impair the correct functioning of the device
and therefore the eectiveness of the application in the long term.
5. Cleaning and Disinfection Method
After each use, it is necessary to clean and disinfect the cup
components (including medication cup, lid), spray assem-
bly, mask or mouthpiece. The specific methods of cleaning
and disinfection are recommended as follows:
5.1. Cleaning:
Please turn o the power when cleaning device. Do not connect the device with
power supply.
1) Remove the components from the main unit: the cup components (including
medication cup,lid), spray assembly, mask or mouthpiece, and soak all the
components the main unit excluding in clean warm water (which is no more
than 40°C) for about 5 minutes.
2) After cleaning, wipe all the components with clean medical gauze, and leave
on open air to dry completely.
3) Wipe the outer shell of main unit. If there is medicine residue remaining at the
electrode contact, please clean it with wet clean medical gauze. After cleaning,
keep the main unit dry.
4) Store all the parts in a dry and clean place to avoid contamination.
Note:
· The main unit can not be washed with water to prevent water from entering
the main unit.
· Use clean medical gauze to wipe the moisture of the main unit and compo-
nents and keep them dry to ensure safe use next time.
• The masks must not be placed in hot water!
5.2. Disinfection