gce mediline MEDIREG II User manual

MEDIREG® II, MEDISELECT® II
HIGH PRESSURE REGULATORS
REGULADORES DE ALTA PRESIÓN
REDUTORES DE PRESSÃO
RIDUTTORI DI PRESSIONE
DÉTENDEURS HAUTE PRESSION
INSTRUCTION FOR USE
INSTRUCCIONES DE USO
INSTRUÇÕES DE UTILIZAÇÃO
MANUALE DUSO
MANUEL DUTILISATION
EN
ES
PT
IT
FR


MEDIREG® II, MEDISELECT® II
English ..............................2
Español..............................13
Português ...........................25
Italiano .............................37
Français .............................49

EN
2/60
FOREWORD
GCE Medical Regulators are medical devices classied as class IIb according
to the Medical Device Directive 93/42/EEC.
Their Compliance with essential requirements of 93/42/EEC Medical Device
Directive is based upon EN 10524-1 standard.
INTENDED USE
GCE Medical Regulators are designed for use with high-pressure medical
gas cylinders equipped with a medical cylinder valve. They regulate pres-
sure and ow of medical gases to the patient. They are intended for the ad-
ministration of the following medical gases in the treatment, management,
diagnostic evaluation and care of the patient:
• Oxygen;
• Nitrous oxide;
• Air for breathing;
• Helium;
• Carbon dioxide;
• Xenon;
• Specied mixtures of the gases listed.
• Air or nitrogen to power surgical tools.
OPERATIONAL, TRANSPORT AND STORAGE
SAFETY REQUIREMENTS
Keep the product and its associated equipment away from:
• All sources of heat,
• Flammable materials,
• Oil or grease (including all hand creams),
• Water,
• Dust.
The product and its associated equipment must be prevented from fall-
ing over.
Always maintain oxygen cleanliness standards,
Use only the product and its associated equipment in a well ventilated
area.
1.
2.
3.
ENGLISH
INSTRUCTION FOR USE: MEDIREG® II, MEDISELECT® II

EN
3/60
Before initial use the product should be kept in its original packaging. GCE
recommends use of the original packaging (including internal sealing bag
and caps) if the product is withdraw from operation (for transport, storage).
Statutory laws, rules and regulations for medical gases, accident prevention
and environmental protection must be observed.
Operating conditions Storage and transport
conditions
-20/+60 °C -30/+60 °C
10/100% 10/100%
600/1200 mbar 600/1200 mbar
In case of storage at a temperature below -20 °C, d o not operate the reg-
ulator until it has been allowed to increase its temperature to a minimum
of -20 °C.
At regulators intended for use with mixture of medical gases O2+N2O is
lower operation temperature limit +5°C. During normal use the ow out-
let and pressure outlet will sometimes have a frosty appearance. This is
a normal physical reaction in the valve, due to that the gas is going from
high pressure to low pressure (Joule Thompson eect). Ensure that all
equipment that is connected to the valve by at least a 2 metre hose.
PERSONNEL INSTRUCTIONS AND TRAINING
According to Medical Devices Directive 93/42/EEC the product provider
must ensure that all personnel handling the product are provided with the
operating instructions & performance data and are fully trained to carry out
that operation. Trainees need to be supervised by an experienced person.
Do not use the device without being trained. Training can be only done
by a person with an appropriate education, experience and knowledge
that has been also trained by the manufacturer.
For further information about training programs, please contact GCE.
4.

EN
4/60
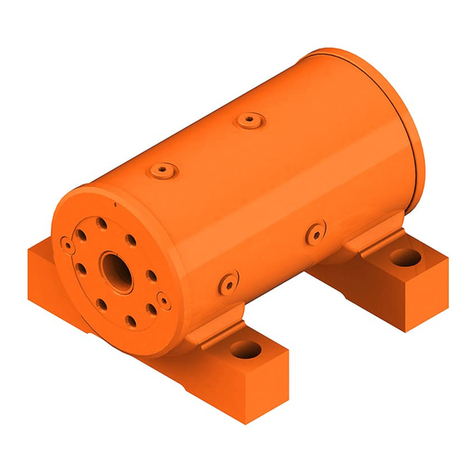
PRODUCT DESCRIPTION
The regulator acts as a pressure-reducer, gas from the cylinder valve passes
through the pressure regulator to the user outlets.
E
B
B
E
A
A
C
F
D
Conguration of MediReg® II regulator
with owmeter
Typical conguration of MediSelect®II
regulator
A - Inlet stem
Regulator is tted to the medical cylinder valve by mean of an inlet stem.
The stem can be bull nose type (male thread), nut type (female thread) or
pin index type. The inlet stem includes a lter.
B - Inlet pressure indicator or sensor
The regulator is tted with a pressure indicator or sensor which is intended
for cylinder gas content indication only, not for measuring purposes. The
pressure indicator or sensor can be equipped with output of electric signal.
The connection of pressure indicator with output of electric signal must be
carried out by trained personnel in accordance with national regulations
pertaining to the electric device and standard EN ISO 7396-1.
Output of electric signal shall be connected only to device which is in ac-
cordance with standard EN ISO 60601-1 and 60601-1-2.
C, D, E – Flow-metering device and ow outlet
GCE regulators can be supplied with a ow-metering device - ow control
head “C” or owmeter “D”. This function is used to supply a gas ow (l/min)
at atmospheric pressure directly to the patient through the ow outlet “E”,
e.g. through a cannula or a facemask.
The ow outlet “E” can be hose nipple (for cannula or mask) or outlet with
thread (for humidier).
5.

EN
5/60
F - Pressure outlet
The regulator may be tted with a pressure outlet.The pressure outlet is the
outlet directly from the low-pressure chamber. Two types of the pressure
outlet can be used:
Pressure outlet I - is tted with a gas specic medical quick connector also
called “quick coupler” . The user can connect another piece of equipment
to this outlet with a gas specic male probe. The quick connector self seals
when the male probe is disconnected. This outlet is for supplying gas at a
controlled pressure to power medical devices, e.g. medical ventilator.
Pressure outlet II – is tted with a threaded connector. The regulator with
this type of pressure outlet shall be only an integral part of a medical equip-
ment (e.g. emergency ventilators, anaesthesia devices, etc.)
If the regulator is tted with two pressure outlets, do not use both of
them at the same time. If you use both of them in the same time the per-
formance of the regulator will not be according to specication (see ap-
pendix 1) !!!
Note also that the product colour (especially ow control knob) might
not be gas specic colour coded.
OPERATIONS
6.1. BEFORE USE
6.1.1. Visual Inspection before use
• Check if there is visible external damage to the product (including prod-
uct labels and marking) and on the gas cylinder. If it shows signs of exter-
nal damages, remove it from service and identify its status.
• Visually check if the product or the medical gas cylinder is contaminated;
if needed, for the regulator, use the cleaning procedure detailed in this
section (if required for the cylinder, refer to the gas cylinder manufacturer
cleaning recommendation).
• Check that the total life time of the product and the gas cylinder has not
been exceeded, (refer to GCE or owner’s date coding system). If life time
has been exceeded, remove the product (or the gas cylinder) from service
& suitably identify its status.
• Ensure that the product inlet stem is compatible with the medical cylin-
der valve (gas/ thread type).
• Check the presence & the integrity of inlet stem seals / correct size of seal.
Always make sure the inlet stem o-ring is in good status, without damage.
Remove caps from inlet and/or ow outlet. Keep caps in a safe place for
reuse during transport or storage.
The product is dedicated only for use with the gas specied on its label-
ling. Never try to use for another gas.
6.

EN
6/60
6.1.2. Fitting to medical cylinder valve
• Secure the gas cylinder stand.
Screw connection (bull nose or nut type)
• Connection equipped with rubber sealing - tighten by hand!
• Connection equipped with metal to metal sealing or plastic sealing -
tighten by means of a torque wrench (max. tightening torque is 50Nm).
• Turn the regulator into the correct position for use and tighten the nut by
hand - do not use tools.
Pin index connection
• Position the pin-index over the cylinder valve with the pin(s) on the pres-
sure regulator pointing towards the cylinder valve connector holes on
the cylinder valve.
• Press the regulator inlet connection pins into the cylinder valve connec-
tor holes - do not use force, otherwise the pins or holes may be damaged.
• Tighten the screw on the regulator onto the cylinder valve connector via
the T-bar handle. Do not use tools.
• Position the equipment so that the regulator user outlets point away
from personnel or patient.
Fitting the regulator with too high a torque to the cylinder valve may re-
sult in damage.
During tting to the cylinder valve, do not apply torque/load to any other
parts of the product.
6.1.3. Leakage check before use
• For regulators tted with a ow-metering device, set the ow control
knob on the “ZERO” position - Ensure the ow control knob engages cor-
rectly.
• Open the cylinder valve slowly by turning the hand wheel in anticlock-
wise direction approx 1 to 1½ turns.
Sudden opening of the cylinder valve could result in a danger of re or
explosion arising from oxygen pressure shocks. Insucient opening of
the cylinder valve could reduce actual ow delivered.
• Perform visual and audible check for possible leakages:
• regulator inlet connection to cylinder valve
• pressure indicator/sensor to regulator body
• pressure relief valve vent hole(s)
• owmeter (if any)
• Turn o the cylinder valve by turning the hand wheel in an clockwise di-
rection to stop position. Do not use excessive force.
If any leakage is detected, use the procedure in chapter 6.3 and return
the product to GCE for service.

EN
7/60
6.1.4. Functional checks before use
• Ensure the ow control knob is on the “ZERO” position.
• Ensure the cylinder valve is open – in the“ON”position.
• Check that the gauge indicates pressure/contents. If the pointer reaches
the red area send the cylinder for the lling
• For regulators tted with a ow-metering device check that there is gas
ow at each setting (for instance, by listening for the sound of gas ow or
checking presence of bubbles in a humidier).
• Turn o the shut o cylinder valve by turning the hand wheel in a clock-
wise direction to the stop position. Do not use excessive force.
• Reset the ow control knob to on the “ZERO” position once the gas ow
stops and the regulator is vented..
• For regulators tted with a pressure outlet, ensure it is functional by con-
necting and disconnecting a male QC probe.
6.2. USER OUTLETS CONNECTION & USE
6.2.1. List of recognised accessories
To be connected to the ow outlet:
Humidiers, breathing masks or cannulas, gas savers, nebulizers.
To be connected to the pressure outlet:
Flexible hoses, ow meters, Venturi suction ejectors.
At regulators tted with pressure outlet together with ejector outlet
don´t use quick coupler and ejector outlet in the same time. Especially
when inlet pressure is bellow 50 bar it may negatively aect performance
of the regulator.
Before connecting any accessory or medical device to the regulator, al-
ways check that it is fully compatible with the product connection fea-
tures & the product performances.
6.2.2. Pressure outlet connection
Pressure outlet I
• Ensure the male quick coupler is compatible with the pressure outlet
feature.
• Connect the male quick coupler.
• Check if the male quick coupler is fully engaged.
Regulator with threaded connector as pressure outlet shall be only an
integral part of medical equipment. Do not use it for other purposes!
Pressure outlet II
• Ensure the counterpart is compatible with the pressure outlet features.
• Screw the counterpart.
• Check the counterpart is fully screwed.

EN
8/60
When is pressure outlet used by medical product with high ow con-
sumption (for example lung ventilator with request of source ow
100 l/min at minimal pressure 2,8 bar) check the required capacity
of source device with regulator pressure outlet performance listed in
appendix 1. To obtain enough performance of the regulator is recomend-
ed replace cylinder when gauge reach the red area.
6.2.3. Flow outlet connection
When connecting any accessory to the ow outlet make sure that it is not
connected to the patient before operating the product.
• Ensure the hose/humidier is compatible with the ow outlet feature.
• Push the hose onto the regulator ow outlet/outlet for humidier.
• Ensure the hose/humidier is well engaged.
6.2.4. Use of product through the ow outlet (Flow setting)
• Ensure that the ow control knob is on the ZERO position.
• Ensure that the accessory is connected to the ow outlet.
• Slowly open the cylinder valve by turning the hand wheel in anticlock-
wise direction approx. 1 to 1½ turns.
Sudden opening of the cylinder valve could result in a danger of re or
explosion arising from oxygen pressure shocks. Insucient opening of
the cylinder valve could reduce actual ow delivered.
• Set the ow control knob on the required one of the available ow rates.
Always ensure that the ow control knob has engaged and not placed
between two settings otherwise the ow selector will not deliver the cor-
rect ow of medical gas.
Do not try to apply an excessive torque on the ow control knob when it
stops on the maximum ow position or in zero position.
The oxygen ow rate must be prescribed and administered by a clinically
trained user.
After completion of the therapy
• Turn o the cylinder valve by turning the hand wheel in a clockwise direc-
tion to stop position. Do not use excessive force.
• Vent gas pressure from downstream equipment.
• Reset ow control knob on the ZERO position when gas venting has
ceased.
• Disconnect the tube/humidier from the ow outlet.

EN
9/60
6.2.5. Use of product through the pressure outlet.
• Ensure that the ow control knob is on the ZERO position (if any).
• Ensure the accessory IS NOT connected to the pressure outlet.
• Slowly open the cylinder valve by turning the hand wheel in anticlock-
wise direction approx 1 to 1½ turns.
Sudden opening of the cylinder valve could result in a danger of re or
explosion arising from oxygen pressure shocks. Insucient opening of
the cylinder valve could reduce actual ow delivered.
• Connect the accessory to the pressure outlet.
After completion of the therapy
• Turn o the cylinder valve by turning the hand wheel in a clockwise direc-
tion to the stop position. Do not use excessive force.
• Vent gas pressure from downstream equipment.
• Disconnect the male QC probe from the pressure outlet.
6.3. AFTER USE
• Turn o the cylinder valve by turning the hand wheel in a clockwise direc-
tion to the stop position. Do not use excessive force.
• Reset the ow control knob on the “ZERO” position – when the gas vent-
ing has ceased (valid for version with ow-metering device only).
• Ensure the pressure indicator does not show any residual pressure.
• Remove connections from user outlets.
• Ret pressure outlet and ow outlet protection caps. Before retting the
caps, ensure they are clean.
CLEANING
Remove dirt with a soft cloth damped in oil free soap water & rinsed with
clean water. Disinfection can be carried out with an alcohol-based solution
(with damped wipes). If other cleaning solutions are used, check that they
are not abrasive and they are compatible with the product materials (in-
cluding labels) and gas (convenient cleaning solution - i.e. Meliseptol)
Do not use cleaning solutions containing ammonia!
Do not expose to water or any other liquid.
Do not expose to high temperature (such as autoclave).
To apply the cleaning solution do not spray it as the spray may enter into
the inner parts of product and cause contamination or damage.
Do not use pressure wash as it could damage or contaminate the pro-
duct.
If the inner parts of the product have been contaminated do not con-
tinue to use the product under any circumstances. It must be withdrawn
from service.
7.

EN
10/60
MAINTENANCE
8.1. SERVICE AND PRODUCT LIFE TIME
8.1.1. Serial number and date of production
Form of nine digit serial number stamped on the product is following:
YY MM XXXXX
YY: year of production;
MM: month of production;
XXXXX : sequence number
Example: serial number 090300521 shows the regulator produced in March
2009, with sequence number 521.
8.1.2. Maintenance
No special maintenance or service, apart from the tests before use, is need-
ed. However, to make sure the product is in good working order, it would
be good if the owner/cylinder distributor performs the checks (see 6.1) him-
self on a regular basis (ex. once every second year) and/or at every cylinder
exchange. This is just for the owner to ensure that the product works well,
especially in cases where the user has some health problems and is not able
to check the product himself properly.
8.1.3. Maximum life time and waste management
Maximum life time of the product is 10 years from the manufacturing date.
At the end of the product’s life time (10 years maximum), the product must
be withdrawn from service. The provider of the device shall prevent the re-
use of the product and handle the product in compliance with“Directive of
European Parliament and Council 2006/12/ES of 5th April 2006 on waste“.
8.2. REPAIRS
8.2.1. Repairs
Repairs activities cover the replacement of the following damaged or
missing components:
• Inlet stem,
• Flow-metering device,
• Indicator,
• Piston,
• Pressure relief valve,
• Quick coupler.
The repairs shall be carried out by a GCE authorised person only.
Any product sent back to a GCE authorised person for maintenance shall
be properly packaged. The purpose of the maintenance has to be clearly
specied (repair, overall maintenance). For product to be repaired a short
description of fault and any reference to a claim number might be helpful.
8.

EN
11/60
Some repair activities concerning to the replacement of the damaged or
missing components can be carried out by the owner of the product. The
following parts can be replaced only:
• Caps,
• Flow knob and stickers,
• Hose nipple (including o-ring)
• Inlet stem o-ring.
Contact our customer service for appropriate component number
All labels on the equipment must be kept in good, legible condition by
the owner and the user during the entire product life time.
All seals and o-rings must be kept in dry, dark and clean environment by
the owner and the user during the entire product life time.
Use only original GCE components!
GLOSSARY
Consult instruction for use Suitable for Home care use
Caution Suitable for Hospital care
use
Keep away from heat and
ammable material
Suitable for Emergency
care use
Keep away from oil and
grease SN Serial number
Upper and lower humidity
limit REF Reference number
Upper and lower tempera-
ture limit LOT Batch number
Keep dry Fragile
Date of manufacture Manufacturer
Use by date Weight of product
Inlet parameter Outlet parameter
9.

EN
12/60
P1Inlet pressure range P2Outlet pressure
P4
Max outlet pressure (clos-
ing pressure) QOutlet ow
Take back equipment for
recycling. Do not dispose
into unsorted municipal
waste.
Ambient pressure limit
WARRANTY
The Standard Warranty period is two years from date of receipt by the GCE
Customer (or if this is not known 2 years from time of the product manufac-
ture shown on the product).
The standard warranty is only valid for products handled according to In-
struction for use (IFU) and general industry good practice and standards.
10.
0434
Appendix:
Nr 1- Technical and performance data
Nr 2 - Quick coupling feature and connecting/disconnecting procedure
Manufactured by:
GCE s.r.o. Tel : +420 569 661 111
Zizkova 381 Fax : +420 569 661 602
583 81 Chotebor http://www.gcegroup.com
Czech Republic © GCE s.r.o.

ES
13/60
INTRODUCCIÓN
Los reguladores GCE son dispositivos médicos de clase IIB conforme a la
directiva sobre los dispositivos médicos 93/42/EEC.
La conformidad del producto con los requerimientos esenciales de la direc-
tiva 93/42/CEE está basada sobre la Norma EN10524-1.
USO PREVISTO
Los reguladores están destinados para ser conectados a cilindros de alta
presión provistos de una válvula de cilindro. Regulan la presión y el ujo de
gases medicinales. Están destinados para el uso en rescate, primeros auxi-
lios, diagnóstico y terapias hospitalarias y domiciliarias con los siguientes
gases medicinales:
• Oxígeno (O2)
• Protóxido, u óxido nitroso
• Aire medicinal
• Helio (He)
• Dióxido de carbono (CO2)
• Xenón
• Mezclas de los gases arriba mencionado
• Aire y Nitrógeno (N2) para la alimentación de herramientas quirúrgicas
REQUERIMIENTOS OPERACIONALES, DE
TRANSPORTE Y DE SEGURIDAD DE ALMACENAJE
Mantenga el producto y sus accesorios alejados de:
• Fuentes de calor (fuego, cigarrillos, …),
• Materiales inamables,
• Aceite o grasa, (cuidado con el uso de crema de manos)
• Agua,
• Polvo.
El producto y sus accesorios deben estar protegidos contra posibles caí-
das.
Mantener siempre los estándares de limpieza para el oxígeno.
Usar el producto y sus accesorios únicamente en áreas bien ventiladas.
1.
2.
3.
ESPAÑOL
INSTRUCCIONES DE USO : MEDIREG® II, MEDISELECT® II

ES
14/60
Durante el almacenaje, antes del primer uso, el producto debe estar en su
embalaje original termo sellado. En caso de ser retirado de servicio para su
transporte o almacenaje, GCE recomienda usar el mismo embalaje original
(incluso los materiales de protección).
Es necesario observar las leyes nacionales, los reglamentos y regulaciones
para gases medicinales, la prevención de accidentes y la protección del me-
dio ambiente.
Condiciones de uso Condiciones de almacenaje y
transporte
-20/+60 °C -30/+60 °C
10/100% 10/100%
600/1200 mbar 600/1200 mbar
En caso de almacenamiento a temperatura inferior a -20 ºC, no usar el
regulador hasta que la temperatura sea superior a -20 ºC.
Los reguladores para mezclas de gases medicinales como O2+N2O la
temperatura limite de uso es de +5 ºC. Durante el uso normal, la salida
tendrá a veces una apariencia congelada, con escarcha. Esto es una reac-
ción física normal, debido a que el gas esta pasando de alta a baja pre-
sión (efecto Joule Thompson). Asegúrese que el equipo esta conectado a
la válvula del paciente con una manguera de al menos 2 m.
INSTRUCCIONES Y CAPACITACIÓN DEL PERSONAL
Según la directiva 93/42/CEE para dispositivos médicos , el propietario del
producto deberá asegurarse, que todos los usuarios tengan las instruccio-
nes de uso, los datos técnicos a su disposición y que estén perfectamente
calicados para realizar las operaciones respectivas con el dispositivo. Las
capacitaciones deberán ser supervisadas por personas calicadas.
No utilice el dispositivo sin haber sido capacitado. La capacitación debe
ser dada por una persona con la apropiada educación, experiencia y co-
nocimiento y también debe haber sido entrenado por el fabricante.
Para obtener más información sobre los programas de capacitación, por fa-
vor póngase en contacto con la GCE.
4.

ES
15/60
DESCRIPCIÓN DEL PRODUCTO
La válvula reguladora reduce la presión de gas. El gas pasa por la válvula de
cilindro a través del regulador hasta llegar a las salidas de uso.
E
B
B
E
A
A
C
F
D
La conguración de la válvula
reguladora MediReg®II
con el caudalímetro)
La conguración típica
de la válvula reguladora
MediSelect® II)
A - Conexión de entrada
La válvula reguladora está unida a la válvula cilindro mediante la conexión
de entrada. La conexión de entrada puede tener una tuerca con rosca inter-
na, tuerca con rosca externa u el estribo. Dentro de la conexión de entrada
se encuentra el ltro.
B - Manómetro o indicador de presión de entrada
El regulador viene equipado con un manómetro o indicador de presión que
indica únicamente el contenido del cilindro.
El indicador de presión puede ser equipado con una señal eléctrica en la
salida. La conexión debe ser hecha únicamente por personal debidamente
formado y siempre respetando los reglamentos nacionales pertinentes a los
dispositivos eléctricos y la norma EN ISO 7396-1.
Salida de la señal eléctrica se deberá conectar únicamente a dispositivos
conformes a la norma EN ISO 60601-1 y 60601-1-2.
C, D, E – Caudalimetro y la salida de ujo
Los reguladores pueden contemplar un mando de control de Flujo – el cau-
dalímetro integrado “C” o el caudalímetro “D”. Esta función está destinada
para asegurar un Flujo de gas (l/min) a presión atmosférica directamente
al paciente mediante la salida del caudal “E”, por ejemplo, a través de una
cánula o de una mascarilla de oxígeno.
5.

ES
16/60
El conector de salida de caudal„E“ puede ser de tipo espiga para cánula,ga-
fas de oxigenoterapia o máscara, también se suministra versiones con una
conexión roscada para el frasco humedecedor.
F - Toma de salida de presión
Los reguladores pueden ser previstos de una salida de presión. La salida de
presión es la salida directa desde la cámara de presión baja. Pueden aplicar-
se dos tipos de salidas:
Salida de presión I – está equipada de una toma de presión especica al gas,
llamado también„acople rápido“. La toma de presión permite
conectar otros aparatos mediante el conector especíco para el gas dado.
Cuando la toma de presión está desconectada, la salida de presión se corta
automáticamente. Esta salida está destinada para el suministro de gas con
presión regulada para el abastecimiento de dispositivos médicos, como
ventiladores, incubadoras y otros.
Salida de presión II – equipada de una conexión roscada sin check. Los re-
guladores con este tipo de la salida de presión, solo pueden formar parte
de un dispositivo médico (por ejemplo el ventilador médico, máquina de
anestesia etc.).
¡¡¡Si el regulador esta equipado de 2 salidas de presión, no usar a la vez
ambas salidas. Si usa las 2 salida a la vez, el caudal que puede proveer el
regulador no será de acuerdo con la especicación (véase el anexo No. 1)
Nota: El color del producto (especialmente el volante de ajuste de
ujo) puede no corresponder a la codicación de color de gas para
facilitar su uso en aplicaciones de emergencias o domiciliaria.
OPERACIONES
6.1. ANTES DEL USO
6.1.1. Inspección visual antes del uso
• Compruebe que no existe ningún daño en el regulador, su conexión o en
la válvula de cilindro (incluso en las etiquetas y la marcación). En caso de
daño, no usar el producto: contactarnos, o, contactar su proveedor local
o poner el producto fuera de servicio e indique debidamente su estado.
• Compruebe visualmente si existen contaminaciones (presencia de grasa
o suciedad) del regulador y de la válvula de cilindro; aplique el proce-
dimiento de limpieza indicado más adelante, o, contáctenos, o también
puede contactar su proveedor local en caso de visualizar algún tipo de
contaminación.
• Compruebe que el tiempo de vida total del regulador y del cilindro no se
haya excedido (ver el sistema de codicación de GCE o de su proveedor
local). Ponga el producto fuera en el caso de haber sobrepasado dicho
tiempo de vida útil e identique debidamente su estado.
6.

ES
17/60
• Vericar si el conector de entrada es compatible con la válvula de cilindro
de gas medicinal (gas/tipo de rosca).
• En caso de ser equipado, vericar la presencia y el buen estado de las
juntas (o´ring) de la conexión de entrada. Asegúrese que la junta tórica
del vástago de entrada se encuentra siempre en buen estado, sin daños
aparentes.
Remueva (quita) el tapón protector de la conexión de entrada y/o de la
salida de ujo. Guarde los tapónes en un sitio limpio y seguro para un uso
posterior para su transporte o almacenaje.
El producto está destinado para ser usado con el gas indicado sobre su
etiqueta. Jamás utilizarlo con otro gas.
6.1.2. Montaje de regulador sobre la válvula de cilindro
• Ubicar el cilindro en posición vertical y asegurarlo para impedir posibles
caídas.
Conexión roscada (tuerca hexagonal)
• Enroscar manualmente el regulador sobre la válvula de cilindro.
• Posicionar el regulador a la posición correcta de uso.
• Apretar con llave de boca ja sin sobrepasar el torque de 50Nm.
Conexión roscada (tuerca de apriete manual, con muescas, sin hexa-
gonal)
• Enroscar manualmente el regulador sobre la válvula de cilindro.
• Posicionar el regulador a la posición correcta de uso, y apriete la tuerca
manualmente – no utilicen las herramientas.
Conexión de yugo (Pin Index)
• Pongan el estribo sobre la válvula, ajuste las clavijas del estribo contra los
agujeros en la válvula del cilindro, sin forzar.
• Atornillen rmemente el regulador mediante el manubrio -T sobre la vál-
vula del cilindro. No utilicen herramientas.
• Posicione el regulador para que las salidas de uso no estén orientadas
hacia (apuntando) el paciente.
Apretar la conexión del regulador a la válvula de cilindro demasiado
fuerte causa daños al los roscados de las válvulas de alta presión, al regu-
lador y aumenta el riesgo de compresión adiabatica.
Realizando la conexión de la válvula del cilindro no utilicen para el aprie-
te otros componentes del producto.

ES
18/60
6.1.3. Prueba de fugas antes del uso
• Para reguladores con caudalímetro, gire el mando de control de ujo a su
posición“0” - asegúrese que el mando trabaje correctamente.
• Abra lentamente la válvula del cilindro girando en dirección contraria al
reloj aproximadamente de 1 a 1 1/2 vueltas.
Una apertura rápida o repentina podría provocar un peligro de fuego
o de explosión debido a choques de presión de oxigeno. Una apertura
escasa de la válvula de cilindro puede reducir el caudal real entregado.
• Realice una comprobación visual y sonora (posibles ruidos) para detectar
posibles fugas:
• La conexión de entrada del regulador a la válvula del cilindro.
• Indicador/sensor de presión al cuerpo del regulador de presión,
• Oricios de escape de la válvula de seguridad,
• Caudalímetro (si está conectado).
• Girando el cierre manual en dirección del reloj a la posición„stop“ se cie-
rra la válvula del cilindro. No apliquen demasiada fuerza.
En el caso de detectar alguna fuga, aplique el procedimiento inscrito en
el capítulo 6.3 y devuelva la válvula para su servicio.
6.1.4. Prueba funcional antes del uso
• Poner el volante de ajuste de ujo en posición“0”.
• Abre la válvula del cilindro – posición“ON”.
• Verique si el manómetro indica la presión de cilindro. Si la aguja alcanza
el area roja, envié el cilindro a llenar
• Para reguladores caudalímetros, compruebe que haya ujo de gas en
cada posición (por ejemplo, a través del sonido o la presencia de burbujas
en el frasco humedecedor).
• Cierre la válvula del cilindro girando hacia el sentido del reloj hasta su
posición“stop”. No utilice una fuerza excesiva.
• Gire de nuevo el volante de ajuste de ujo a su posición “0” cuando no
haya ningún ujo más - asegúrese que el regulador de ujo encaje bien.
• Para las válvulas reductoras con la salida de presión, compruebe que fun-
cione correctamente, conectando y desconectando un conector (adapta-
dor) de conexión rápida.
This manual suits for next models
1
Table of contents
Languages:
Other gce mediline Controllers manuals