HeartSciences MyoVista Wavelet ECG User manual

Fast Find Links
Introduction .............................1
Safety Summary..........................4
General Overview .......................15
MyoVista Operations .....................28
Patient Testing ..........................36
Device Settings..........................63
Troubleshooting.........................99
Service, Maintenance and Cleaning. . . . . . 103
MyoVista® Wavelet ECG (wavECG™)
12-Lead Cardiac Testing Device
User Manual
Soware Version 2.0
Hardware Version 002

ii
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
NOTICE: YOU HAVE PURCHASED THE MYOVISTA
PRODUCT EXCLUSIVE OF THE SOFTWARE THAT
IS INCLUDED INTERNAL TO THE PRODUCT OR
IS OTHERWISE SUPPLIED FOR USE WITH THE
PRODUCT, AND ANY DATA INCLUDED THEREWITH.
THAT SOFTWARE AND DATA IS OWNED BY
HEARTSCIENCES, USA, AND IS LICENSED TO YOU.
A COPY OF THE END USER LICENSE AGREEMENT
(EULA) AT THE DATE OF THIS DOCUMENT IS SET
OUT IN APPENDIX F OF THIS USER MANUAL. THE
EULA MAY BE UPDATED FROM TIME TO TIME AND
YOU SHOULD REFER TO THE HOME SCREEN OF
THE MYOVISTA DEVICE FOR THE EULA ASSOCIATED
WITH YOUR VERSION OF THE SOFTWARE (YOUR
EULA). YOU SHOULD READ AND UNDERSTAND
YOUR EULA WHICH BINDS YOU CONTRACTUALLY.
IF YOU DO NOT AGREE WITH YOUR EULA DO NOT
OPERATE THE PRODUCT.
THE DOCUMENTS AND RELATED GRAPHICS
MAY INCLUDE TECHNICAL INACCURACIES OR
TYPOGRAPHICAL ERRORS. PERIODICALLY,
CHANGES ARE ADDED TO THE INFORMATION
HEREIN. HEARTSCIENCES, AND/OR ITS RESPECTIVE
SUPPLIERS MAY MAKE IMPROVEMENTS AND/OR
CHANGES IN PRODUCT(S) AND/OR PROGRAM(S)
DESCRIBED HEREIN AT ANY TIME.
THIS DOCUMENT CONTAINS PROPRIETARY
INFORMATION OF HEARTSCIENCES AND ITS
RECEIPT AND POSSESSION DOES NOT CONVEY ANY
RIGHTS TO REPRODUCE, DISCLOSE ITS CONTENTS,
OR MANUFACTURE, USE, OR SELL ANYTHING IT
MAY DESCRIBE OR REPRESENT. REPRODUCTION,
DISCLOSURE, OR USE OF ANY SUCH INFORMATION
WITHOUT SPECIFIC LICENSE OR WRITTEN
AUTHORIZATION IS STRICTLY FORBIDDEN. THE
PUBLICATION OF THIS DOCUMENT BY ANATEL IN
BRASIL IS PERMITTED WHEN REQUIRED BY ANATEL.
HEARTSCIENCES, AND/OR ITS RESPECTIVE
SUPPLIERS MAKE NO REPRESENTATIONS
ABOUT THE SUITABILITY OF THE INFORMATION
CONTAINED IN THIS DOCUMENT AND RELATED
GRAPHICS PUBLISHED. ALL SUCH DOCUMENTS
AND RELATED GRAPHICS ARE PROVIDED
“AS IS” WITHOUT WARRANTY OF ANY KIND.
HEARTSCIENCES AND/OR ITS RESPECTIVE
SUPPLIERS HEREBY DISCLAIM ALL WARRANTIES
AND CONDITIONS WITH REGARD TO THIS
INFORMATION, INCLUDING ALL WARRANTIES
AND CONDITIONS OF MERCHANTABILITY,
WHETHER EXPRESS, IMPLIED OR STATUTORY,
FITNESS FOR A PARTICULAR PURPOSE, TITLE,
AND NON-INFRINGEMENT. IN NO EVENT SHALL
HEARTSCIENCES, AND/OR ITS RESPECTIVE
SUPPLIERS BE LIABLE FOR ANY SPECIAL, INDIRECT
OR CONSEQUENTIAL DAMAGES OR ANY DAMAGES
WHATSOEVER RESULTING FROM LOSS OF USE,
DATA, OR PROFITS, WHETHER IN AN ACTION OF
CONTRACT, NEGLIGENCE, OR OTHER TORTIOUS
ACTION ARISING OUT OF, OR IN CONNECTION WITH
THE USE OR PERFORMANCE OF THE INFORMATION
CONTAINED IN ALL SUCH DOCUMENTS.
All Copyrights © are owned by HeartSciences. All rights are reserved. No part of this publication may be
retransmitted or reproduced in any way, including but not limited to photocopy, photograph, magnetic,
or other record, without prior agreement and written consent of HeartSciences. The publication of this
document by Anatel in Brasil is permitted when required by Anatel.
MyoVista is a trademark of HeartSciences. All trade names are the property of their respective owners.

iii
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Contents
Preface ...................................................................................viii
Preface ............................................................................................................................................................... viii
Audience............................................................................................................................................................ viii
Document Conventions............................................................................................................................... .viii
Typographic Conventions............................................................................................................................ viii
Contacting HeartSciences ..............................................................................................................................ix
Electronic Manual..............................................................................................................................................ix
1. Introduction ........................................................................... 1
Intended Use....................................................................................................................................................... 2
Indications for Use............................................................................................................................................ 2
Contraindications.............................................................................................................................................. 2
Prescription Device Statement..................................................................................................................... 2
Patient Recommendations............................................................................................................................. 3
Patient Condition and History ...................................................................................................................... 3
2. Safety Summary ...................................................................... 4
Safety Conventions........................................................................................................................................... 5
General Warnings and Cautions ................................................................................................................... 5
Warnings ........................................................................................................................................................ 5
Cautions ......................................................................................................................................................... 6
Electrical Safety ........................................................................................................................................... 7
Patient Safety..................................................................................................................................................... 8
Symbols Glossary.............................................................................................................................................. 9
Labeling.............................................................................................................................................................. 12
Device Identification ......................................................................................................................................13
Device Label Format .................................................................................................................................13
Serial Number Format..............................................................................................................................13
Training...............................................................................................................................................................14
Medical Device Classification ...................................................................................................................... 14
3. General Overview ...................................................................15
MyoVista wavECG Device Development ...................................................................................................16
Conventional 12-Lead ECG Waveform ...................................................................................................... 16

iv
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Glasgow Interpretative Analysis ................................................................................................................ 16
MyoVista wavECG Information ...................................................................................................................17
MyoVista Energy Waveform..................................................................................................................... 17
MyoVista Summary Statement and Indicators ..................................................................................18
MyoVista Wavelet Analysis, Algorithms and Statements ................................................................20
Repolarization Measures Algorithm Statement ................................................................................ 21
Ischemic or Structural Risk Assessment Statement (if applicable).............................................. 21
MyoVista Ventricular Indices................................................................................................................... 22
Ventricular Indices Statements (if applicable)...................................................................................23
MyoVista Energy Classification .............................................................................................................. 24
System Overview.............................................................................................................................................25
Front Panel .................................................................................................................................................. 25
Rear Panel....................................................................................................................................................26
Right Side with Patient Cable Port........................................................................................................ 27
Le Side with USB Ports .......................................................................................................................... 27
4. MyoVista Operations ...............................................................28
Getting Started ................................................................................................................................................29
Unpacking the Device............................................................................................................................... 29
Package Contents ...................................................................................................................................... 29
Setting up the MyoVista Device..............................................................................................................29
System Setup and Operation.......................................................................................................................30
Setting Up the MyoVista Device .............................................................................................................30
Initial Soware Configuration................................................................................................................ 32
Built-In Battery...........................................................................................................................................32
Using the Built-in Battery ........................................................................................................................ 34
Monitoring the Battery............................................................................................................................. 34
Maximizing the Battery Life .................................................................................................................... 34
Operating Environment ................................................................................................................................35
5. Patient Testing .......................................................................36
Patient Recommendations...........................................................................................................................37
Patient Condition and History ....................................................................................................................37
Electrode Placement ...................................................................................................................................... 37
Attaching Electrodes to the Patient...................................................................................................... 37
Electrode Placement................................................................................................................................. 38
Electrode Colors.........................................................................................................................................40

v
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Connecting Leads to Electrodes ................................................................................................................. 41
Home Screen.....................................................................................................................................................42
Common Icons............................................................................................................................................43
Keyboard......................................................................................................................................................44
Patient Screen .................................................................................................................................................. 45
Displaying the Patient Screen ................................................................................................................ 45
Adding a New Patient ...............................................................................................................................46
Selecting a Patient.....................................................................................................................................48
Searching for a Patient Record............................................................................................................... 48
Scanning a Patient ID via Barcode Reader.......................................................................................... 49
Editing Patient Information.................................................................................................................... 50
Deleting a Patient Record........................................................................................................................ 50
Test Screen ........................................................................................................................................................51
Displaying the Test Screen ..................................................................................................................... 51
Test Screen Icons .......................................................................................................................................52
Setting Test Options..................................................................................................................................52
Running a Test ............................................................................................................................................54
Re-syncing the Waveforms......................................................................................................................55
Report Screen................................................................................................................................................... 56
Displaying the Report Screen.................................................................................................................56
Report Screen Icons .................................................................................................................................. 57
Printing a Patient Test Report ................................................................................................................58
Selecting Pages ..........................................................................................................................................59
Report Templates ...................................................................................................................................... 59
Report Settings........................................................................................................................................... 60
Exporting a Test Report............................................................................................................................ 61
Deleting a Test Report ..............................................................................................................................62
6. Device Settings ......................................................................63
Settings Screen. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Displaying the Settings Screen ..............................................................................................................64
Identifying the Current Soware and Firmware Versions.............................................................. 64
Settings Screen Icons................................................................................................................................ 65
Configuring System Settings ..................................................................................................................67
Changing the Date/Time Settings .........................................................................................................67
Updating System Soware ..................................................................................................................... 68
Restoring Device to Factory Default Settings..................................................................................... 69
Restoring/Updating Device Reference Materials .............................................................................. 70

vi
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Configuring Network Settings................................................................................................................71
Network Status......................................................................................................................................71
Connecting to a Wired Network............................................................................................................. 71
Connecting to a Wireless Network ........................................................................................................ 72
Setting Up a Printer................................................................................................................................... 73
Database Settings...................................................................................................................................... 75
Viewing the Current Database Information ..................................................................................... 75
Deleting a Database............................................................................................................................. 76
Backing Up a Database....................................................................................................................... 76
Restoring a Database. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
Configuring Auto-Standby Settings .......................................................................................................... 79
Configuring Network Drives ........................................................................................................................80
Configuring Report Settings........................................................................................................................83
Settings Migration Assistant........................................................................................................................84
Export Device Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84
Import Device Settings............................................................................................................................. 86
Export Logs........................................................................................................................................................ 88
EMR Settings .....................................................................................................................................................89
LCD Brightness Settings................................................................................................................................ 90
ECG Filter Settings ..........................................................................................................................................90
Configuring Filters ..........................................................................................................................................90
ECG Trace Colors .............................................................................................................................................. 92
User Settings..................................................................................................................................................... 93
Entering or Modifying the Clinic Information ...................................................................................93
Configuring a Language........................................................................................................................... 94
Configuring the Patient Unit of Measurement...................................................................................95
User Management ........................................................................................................................................... 96
Add User....................................................................................................................................................... 96
Edit User Password....................................................................................................................................98
7. Troubleshooting .....................................................................99
Troubleshooting Table.................................................................................................................................100
8. Service, Maintenance & Cleaning ............................................103
Warnings ..........................................................................................................................................................104
Service & Maintenance ................................................................................................................................104
Maintenance Items to Remember .......................................................................................................104
Cleaning and Disinfecting......................................................................................................................105

vii
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Patient Cable Cleaning...........................................................................................................................105
LCD Screen Cleaning ...............................................................................................................................105
Periodic Inspection.......................................................................................................................................106
Seasonal Safety Check ...........................................................................................................................106
Annual Inspection....................................................................................................................................106
Battery Maintenance....................................................................................................................................107
Supplies and Accessories ............................................................................................................................107
Appendix A. Technical Data .......................................................108
Device Specifications ...................................................................................................................................108
Electrical Specifications..............................................................................................................................108
Environmental Specifications...................................................................................................................108
MyoVista wavECG Specifications .........................................................................................................108
MyoVista Center Post Electrode Specifications ...............................................................................108
ECG Specifications ........................................................................................................................................109
Configuration..................................................................................................................................................111
Data Use ...........................................................................................................................................................111
Potential Issues with Network-Based Printers....................................................................................111
Potential Issues with Network Storage (Mapped Network Drive(s))............................................111
EMC Declaration Tables ...............................................................................................................................112
Electromagnetic Emissions...................................................................................................................112
Electronic Immunity ...............................................................................................................................112
Recommended Separation Distances ................................................................................................113
Appendix B Error Messages ........................................................114
Appendix C VESA Mounting ........................................................115
Appendix D Glossary .................................................................116
Appendix E
MyoVista wavECG Analysis, Algorithms and Statements
......117
Appendix F End User License Agreement ......................................126
Appendix G Limited Warranty.....................................................133
Index ...................................................................................138

viii
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Preface
HeartSciences’ MyoVista® Wavelet ECG (wavECG™) Cardiac Testing Device (MyoVista Device) is a
12-lead resting electrocardiograph (ECG) device. It uses continuous wavelet transform (CWT) signal
processing to provide new energy related information (MyoVista wavECG Information) in addition to
conventional ECG voltage-based information. In addition to the wavECG Information, the MyoVista
Device also features the capabilities of a full featured 12-lead resting ECG including analysis using the
Glasgow Algorithm, one of the world’s most respected interpretive algorithms. The device has a 15.6-
inch high-resolution touchscreen display and incorporates many easy and intuitive to use features
commonly associated with a tablet device.
This User Manual describes how to set up, operate, maintain, clean, and troubleshoot the MyoVista Device.
It includes technical specifications and error messages. An authorized representative should provide initial
installation and training.
Audience
The MyoVista Device is intended for trained medical professionals who use, maintain, clean, and
troubleshoot the system. All are expected to have a working knowledge of medical procedures and
terminology required for evaluating cardiac patients.
MyoVista wavECG Information and the Glasgow Interpretive Analysis are intended to be used with other
relevant patient information and interpreted by a physician.
Document Conventions
In this User Manual HeartSciences’ MyoVista Wavelet ECG (wavECG) Cardiac Testing Device is also
referred to as MyoVista Device, MyoVista wavECG Device, or device and wavECG is used as a precursor to
information, measures, analysis, or results primarily derived using wavelet signal processing.
The following additional conventions are used in the User Manual.
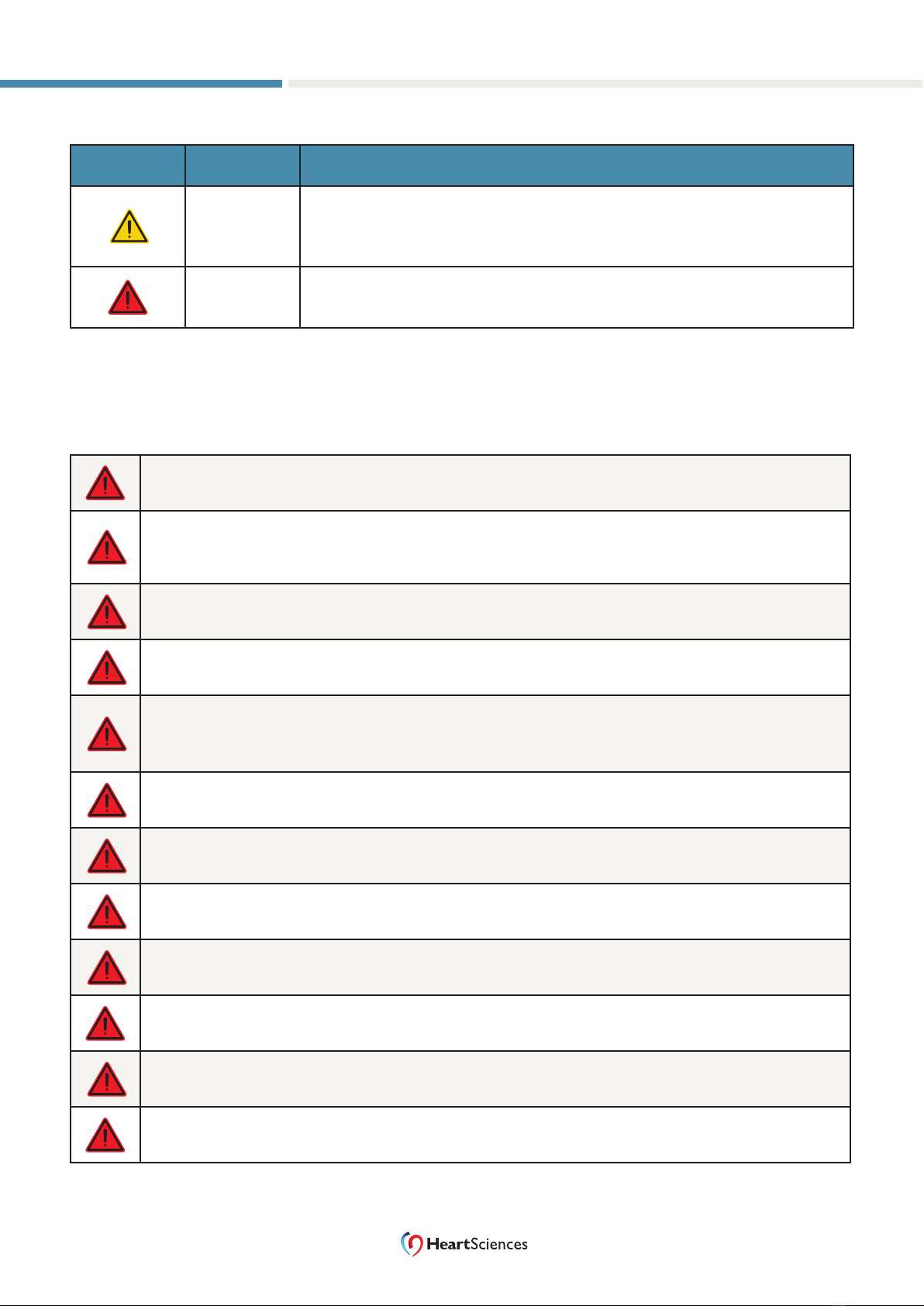
Convention Meaning Description
Note Notes emphasize or supplement important points of the main text.
Tip Tips provide helpful information, guidelines, or suggestions for
performing tasks more eectively.
Typographic Conventions
Convention Description
Bold Indicates soware screen elements including button and icon names, tabs, field names,
dialog box titles, etc. Also used for emphasis.
Italics Indicates document titles.
• Bullets Indicate a list.

ix
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
IMPORTANT
All should read this User Manual carefully before using the medical device.
Contact HeartSciences immediately for any safety concerns.
Contacting HeartSciences
Contact HeartSciences product support directly with inquiries and comments:
support@heartsciences.com
Electronic Manual
This document sets out original instructions from the manufacturer.
To print this manual completely or in part, download the desired PDF files, which can be found on the USB
storage media provided within MyoVista ship container. Follow standard print procedures.
Note: The User Manual cannot be printed directly from the MyoVista Device.
We suggest you print a single page first. You may need to set the printer to Print TrueType Fonts as Bitmaps
(or Graphics) if the character spacing requires correction.

1. Introduction This chapter introduces the MyoVista® wavECG Device
Topics
Intended Use ......................................2
Indications for Use .................................2
Contraindications ..................................2
Prescription Device Statement ......................2
Patient Recommendations ..........................3
Patient Condition and History .......................3

2
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Introduction
Intended Use
The MyoVista® Wavelet ECG (wavECG™) Cardiac Testing Device (MyoVista Device) is a 12-lead resting
electrocardiograph with conventional interpretive analysis of the ECG waveform and MyoVista
wavelet signal processed information.
The MyoVista wavelet signal processed information is intended to be used as an aid in diagnosis by
means of analysis of the ECG waveform in the frequency domain.
Indications for Use
• The MyoVista Device is indicated for use on adult populations (18 years and older), by trained
operators in health facilities as one of the tools used to evaluate and diagnose patient cardiac
function.
• The device is used to acquire, analyze, display, and print conventional 12-Lead ECG information
and provides interpretive analysis and calculations of the QRS, P, and T-wave.
• MyoVista wavelet signal processed information is used to identify patients at risk for LV diastolic
dysfunction as an indicator of heart disease, which may require further anatomical testing.
The interpretations oered by the device are significant only when reviewed by a licensed trained
medical professional and considered in conjunction with all other relevant patient clinical
information.
Contraindications
The MyoVista Device is not indicated for the following uses:
• As a sole means of diagnosis.
• As a vital signs physiological monitor.
• To provide alarms for arrhythmia detection.
• For people whose skin is irritated by electrodes with silver and silver chloride or by the electrode
adhesive.
• For pediatric patients and infants.
Prescription Device Statement
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician or
other licensed practitioner. This device is not FDA cleared.
Non U.S. regional or local laws may restrict the sale of this device to, by, or on the order of a licensed
healthcare practitioner.

3
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Introduction
Patient Recommendations
The following patient factors may aect the accuracy of the MyoVista Device results and should be
considered by the medical professional when interpreting test results:
• Caeine consumption within 2 hours prior to testing.1
• Smoking or using Nicotine replacement therapy.2, 3
• Significant alcohol consumption on the previous day.4
• Phlebotomy within 20 minutes prior to testing.5
• Patients who are not in a horizontal position with a resting heart rate during testing.5
• Patient movement during testing.5
Patient Condition and History
Certain medications can aect test results. Examples include medications taken for heart disease,
hypertension, diabetes (including beta-blockers), digitalis, dobutamine, amildarone, and narcotics.
Prior to testing, review the patient’s history of health issues and medications.
1 Silvio Buscemi, Alessandro Mattina, Maria Rosaria Tranchina, Salvatore Verga, “Acute Eects of Coee on QT Interval in Healthy Subjects”, Nutr J. 2011;
10: 15. Published online 2011 Feb 2. doi: 10.1186/1475-2891-10-15
PMCID: PMC3038145
2 Gepner AD, Piper ME, Leal MA, Asthana A, Fiore MC, Baker TB, et al. (2013) “Electrocardiographic Changes Associated with Smoking and Smoking
Cessation: Outcomes from a Randomized Controlled Trial”. PLoS ONE 8(4): e62311. https://doi.org/10.1371/journal.pone.0062311
3 Ramakrishnan S, Bhatt K, Dubey AK, et al, “Acute electrocardiographic changes during smoking: an observational study”, BMJ Open 2013;3:e002486.
doi: 10.1136/bmjopen-2012-002486
4 Ryan, J M and L G Howes. “Relations between alcohol consumption, heart rate, and heart rate variability in men”, Heart (British Cardiac Society) vol.
88,6 (2002): 641-2.
5 Dekie, L., “High Quality ECG Recording Assurance”, Applied Clinical Trials, July 13, 2017.

Note: Ignoring the provided safety information and intended use
is considered misuse of the MyoVista Device and could result in
harm to the patient, operator, medical facility, or device.
2. Safety Summary
This chapter provides information about the safe use and
regulatory compliance of the MyoVista wavECG Device. You
should be familiar with this information and understand all
instructions before using the device.
Topics
Safety Conventions.................................5
General Warnings and Cautions .....................5
Electrical Safety....................................7
Patient Safety......................................8
Symbols Glossary ..................................9
Labeling ........................................12
Device Identification ..............................13
Training ........................................14
Medical Device Classification .......................14
5
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Safety Conventions
Convention Meaning Description
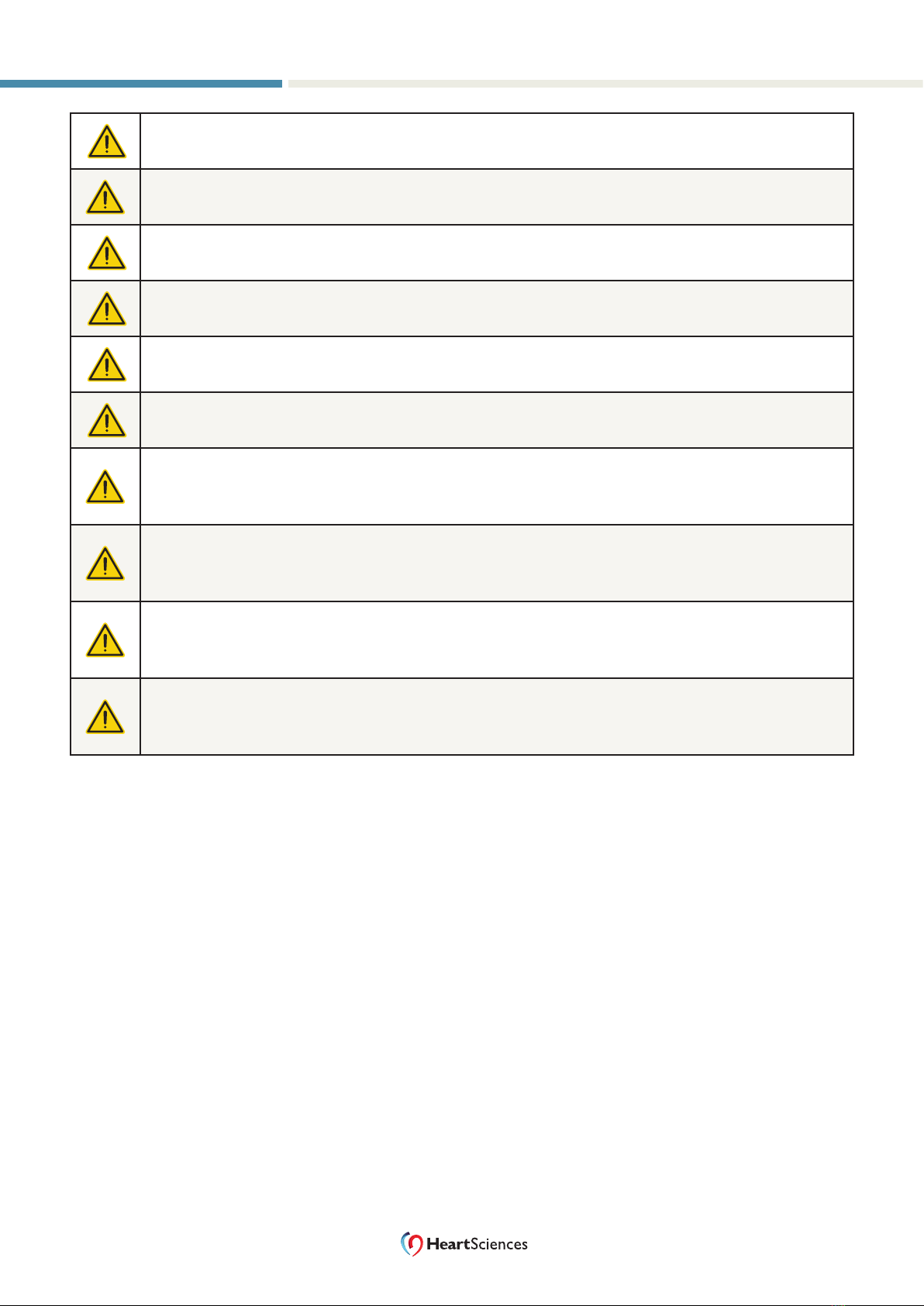
Caution
Indicates a potential hazard or unsafe practice, which, if not avoided,
could result in minor injury, harm to the patient or operator, or damage to
property or the device.
Warning Warnings indicate a potential hazard or unsafe practice, which, if not
avoided, could result in death or serious injury.
General Warnings and Cautions
Warnings
General Warnings in this User Manual are listed below and displayed with the following Warning symbol.
Warning: Operators must read and understand the following safety-related information before
operating the MyoVista Device.
Warning: This is a Class A product. In a domestic environment, this product may cause radio
interference, in which case the user may be required to take adequate measures.
Warning: Operators must read and understand the INTENDED USE, INDICATIONS FOR USE, and
CONTRAINDICATIONS.
Warning: This device may ONLY be used by trained operators under the direct supervision of a
licensed healthcare practitioner.
Warning: DANGER OF ELECTRIC SHOCK. Do not open the device case. Only personnel
authorized by the manufacturer may service the device, open the enclosure, or open the battery
compartment.
Warning: DANGER OF ELECTRIC SHOCK. THERE ARE NO USER-SERVICEABLE PARTS IN THIS DEVICE.
The battery is not user-serviceable.
Warning: DANGER OF ELECTRIC SHOCK. Do not operate the device if the power adapter is
damaged or suspected of being damaged.
Warning: DANGER OF ELECTRIC SHOCK. Do not attempt to repair or alter the device. It can result in
unpredictable operation and risk to the patient.
Warning: The device, accessories, and packaging have safety labels. Operators must be able to
recognize them and understand their meaning (see “Symbols and Icons” on page 9).
Warning: Only cables and electrodes provided by HeartSciences are to be used with the MyoVista
device. Do not use electrodes or cables that have not been approved.
Warning: Do not use electrodes if the backing has been removed or damaged.
Warning: Do not reuse electrodes. The electrodes approved for this device are for single use only.
Using electrodes more than once may lead to inaccurate test results.
Safety Summary

6
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Warning: Never put any cable other than the power adapter into a wall receptacle.
Warning: Never use the device in the presence of flammable vapors or gases.
Warning: Do not use the device beyond the safe limits of environmental conditions of temperature
and humidity (see “Environmental Specifications” on page 108).
Warning: Do not allow liquids to enter the device. Do not have liquids or drinks near the device.
An accidental spill of liquid on the device can create a hazard of electric shock or damage to the
device. If liquid does get into the device, contact an authorized technician before use.
Warning: MyoVista Information and Glasgow Interpretative Analysis must be interpreted by a
licensed healthcare practitioner.
Cautions
General Cautions in this User Manual are listed below and displayed with the following Caution symbol.
Caution: Always confirm the patient’s identification and the information displayed before starting
a test.
Caution: When using the VESA mount interface on the back panel, be careful to check the adequacy
of the surface (for example, the cart, wall, or shelf) to which the device will be attached. The VESA
mount and surface should be able to support 8 kg (17.6 lbs).
Caution: When setting up, be careful to place the device in a stable location, away from the edge of
tables or carts. Avoid entangling the cables with the patient or the operator.
Caution: Do not attempt to reposition the device by pulling the cables. Be careful not to cause the
device to fall by attempting to pull the device by the cables. Always ensure all cables are positioned
properly to avoid damage.
Caution: Patient cable must be inserted properly into the device, with the screws secured tightly to
ensure signal capture.
Caution: The device should be plugged into a grounded outlet with surge protection.
Caution: The device and cables should be cleaned regularly according to instructions.
Caution: Periodically check the battery performance, and have the battery replaced or serviced by
an authorized technician if necessary. For instructions about monitoring the battery, see “Using
the Built-In Battery” on page 34.
Caution: Do not use the device beyond its intended lifetime, which is 7 years from the
manufacturing date.
Safety Summary
7
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Caution: Improper placement of electrodes, patient cable, and leads can provide improper results.
Ensure that correct electrode placement protocol is followed.
Caution: Always use new electrodes from an unopened pouch.
Caution: Do not store or use the device in the presence of strong magnetic fields or ionizing
radiation.
Caution: Do not store or use the device next to instruments that generate strong vibration.
Caution: The patient must be at rest and not moving (other than normal breathing) during the test.
Caution: Always check the quality of the ECG waveforms to ensure the patient connections and the
device have been set up properly before starting the test.
Caution: If poor electrical grounding exists in the clinic, or if a noisy power source is being used,
the device should operate ONLY on battery power when running a test, using the AC power ONLY to
maintain battery charging or non-test use.
Caution: At the end of its service life, the device and its accessories must be disposed of in
compliance with the local governmental guidelines regulating the disposal of medical devices.
Caution: When the low battery warning is displayed, the user should complete their work and then
recharge the device.
Caution: Leaving the device battery fully depleted (0% charge) for a prolonged time may render the
battery nonrecoverable and the device unusable until the battery is replaced (see page 34).
Electrical Safety
Leakage Current: The patient connection interface complies with safety standards for medical device
leakage current levels.
Ensure that the AC power voltage complies with the device requirement.
The power adapter/cord is to be used for disconnecting the device from the electric/mains power
source.
ANSI/AAMI ES60601-1:2005/(R)2012 and A1:2012, C1:2009/(R)2012 and A2:2010/(R)2012
Safety Summary

8
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Safety Summary
Patient Safety
The MyoVista® Wavelet ECG (wavECG™) Cardiac Testing Device (MyoVista Device) meets the
essential requirements of the European Medical Device Directive for General Product Safety
and complies with the applicable U.S., Canadian and other medical safety standards where the
MyoVista Device is registered to be sold.
The design of the equipment, accompanying documentation, and labeling on the equipment
take into consideration that the purchase and use of the MyoVista Device are restricted to trained
operators.
This User Manual excludes warnings of various hazards that are obvious to a medical professional
and operator of this equipment, consequences of product misuse, and potentially adverse eects in
patients with abnormal conditions.
Warning: Do not use the device beyond the safe limits of environmental conditions of temperature
and humidity (see “Environmental Specifications” on page 108).
Warning: Magnetic field: If this device is used in the presence of magnetic resonance imaging (MRI),
the picture resolution of the MRI may be aected. This environment might also interfere with the
operation of the device.
Caution: RF interference: The device conforms to ANSI/AAMI/EN/IEC 60601-2-25:2011; however,
avoid environments with high levels of RF noise.
Caution: Other interference: The presence of an electrocautery device, infrared energy, or
defibrillator may impact the operation of this device.
Note: Electromagnetic interference: This device conforms to ANSI/AAMI/EN/IEC 60601-2-25:2011.
Note: Biocompatibility: All materials that are subject to user or patient contact are of the type
commonly used in a clinical environment.

9
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Safety Summary
Symbols Glossary
Symbols are used to convey warnings, cautions, prohibitions, mandatory actions, or information.
Familiarity with these symbols assists in the safe and proper use and disposal of the equipment.
Table 2-1 describes the symbols and icons that might appear on the device or its packaging. Not all
of the symbols in the table apply to a particular device or its packaging. The table includes (but is not
limited to) standard symbols defined in ISO 15223-1:2016 “Medical devices — Symbols to be used
with medical device labels, labeling and information to be supplied — Part 1: General requirements”
and/or IEC TIR 60878:2015 “Graphical symbols for electrical equipment in medical practice”, and/or
IEC 60417:2002 DB “Graphical symbols for use on equipment”
• Colored symbols indicate there might be a danger, warning, or mandatory action.
• Black symbols provide additional information or might indicate a caution.
Table 2-1. Symbols and Icons on the Device and Its Packaging
Symbol/Icon Description
Manufacturer (might be accompanied by manufacturer name and address)
ISO 15223-1:2016 - 5.1.1
Date of manufacture
ISO 15223-1:2016 - 5.1.3
Caution (consult accompanying documents)
ISO 15223-1:2016 - 5.4.4
Consult Instructions for Use (User Manual)
ISO 15223-1:2016 - 5.4.3
Alternating current
IEC TIR 60878:2015 - 5032
Direct current
IEC TIR 60878:2015 - 5031
Kensington security slot
Universal Serial Bus (USB) interface

10
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Safety Summary
Network interface
IEC 60417:2002 - 5988
Use by date
ISO 15223-1:2016 – 5.1.4
Temperature limit (indicates both upper and lower limits by upper and lower horizontal
lines)
ISO 15223-1:2016 – 5.3.7
Keep dry
ISO 15223-1:2016 – 5.3.4
Device is running on AC power and/or charging
Device is running on DC power; charge level is indicated by battery icon filling and %
charge indicator
Device is connected to a wired network
Device is connected to a wireless (Wi-Fi) network
Standby icon; LED is illuminated when device is in standby mode. LED also indicates
power source and charge level.
Center negative symbol. Indicates output plug (tip) center is Negative (-) and output plug
barrel (ring) is Positive (+).
IEC 60417:2002 - 5926
Symbol for electrical and electronics devices marking for Directive 2012/19/EU. Device,
accessories, and packaging waste must be disposed of properly at end of usage. Local
disposal ordinances and regulations must be followed.
Defibrillation-proof Type CF Applied Part
IEC TIR 60878:2015 - 5336
11
USER MANUAL DO-LBL-06035(L) | MyoVista® Wavelet ECG (wavECG™) 12-Lead Cardiac Testing Device
Safety Summary
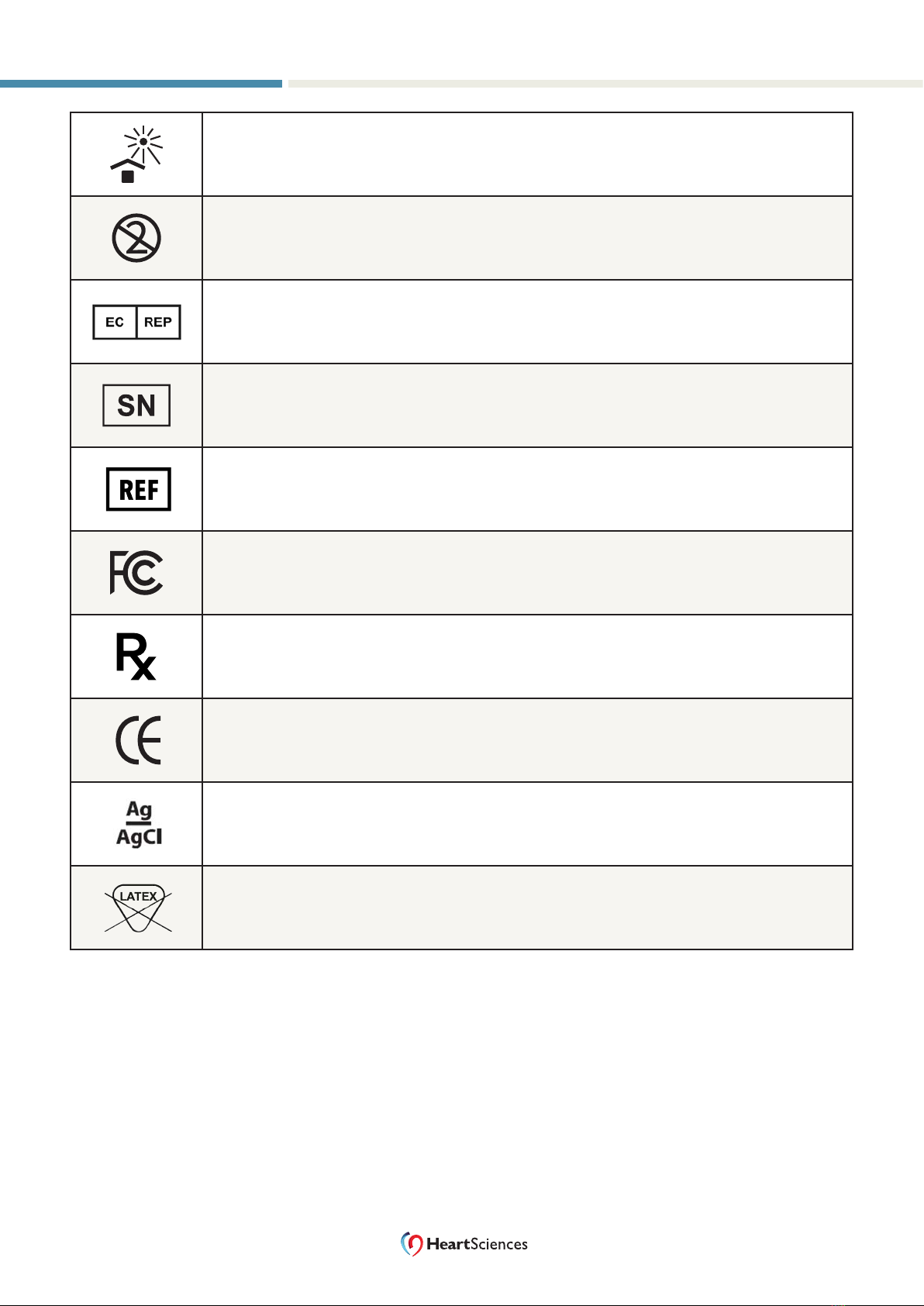
Keep away from sunlight
ISO 15223-1:2016 – 5.3.2
Do not reuse (single use only)
ISO 15223-1:2016 – 5.4.2
European Community authorized representative
ISO 15223-1:2016 – 5.1.2
Serial number
ISO 15223-1:2016 – 5.1.7
Catalog number
ISO 15223-1:2016 – 5.1.6
Meets FCC requirements
USA only: for use by or on the order of a physician
Conforms with applicable EU directives
Contains silver/silver chloride
Latex free - Not made with natural rubber latex
This manual suits for next models
1
Table of contents
Other HeartSciences Test Equipment manuals
Popular Test Equipment manuals by other brands

Keysight Technologies
Keysight Technologies InfiniVision 6000L Series Service guide

Kurth
Kurth KE3700 Short user guide

GE
GE Druck DPI 150 user manual

Dongguan Xin Bao Instrument
Dongguan Xin Bao Instrument XB-OTS-106 Operation manual

Sartorius
Sartorius Sartocheck 3 plus operating instructions

Tektronix
Tektronix TDS 340 manual