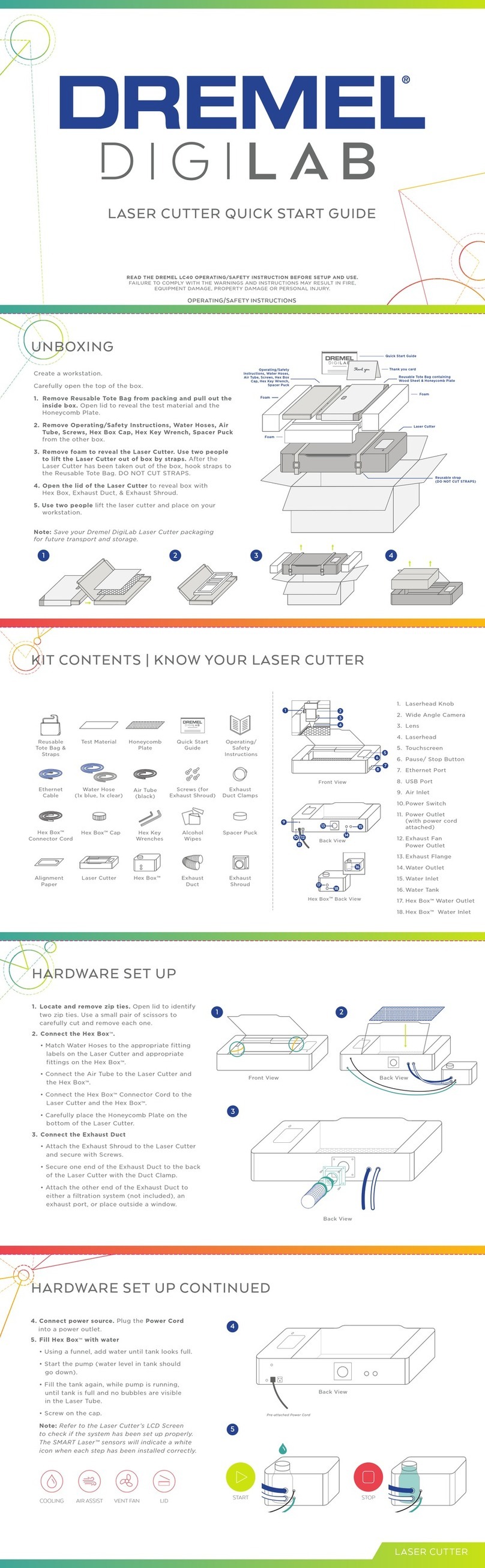
Industrial Test Systems DX-1 User manual

INSTRUCTION MANUAL
for
RDX Nuclear
DX-1
and
DX-2
Radiation Monitors
(Manufactured Since 1982)
INDUSTRIAL TEST SYSTEMS, INC.
MADE in USA
USA
1875 Langston Street
Rock Hill, SC 29730 USA
Phone: (803) 329-9712
Fax: (803) 329-9743
eMail: [email protected]
Web: www.sensafe.com
UK
UK Centre for Homeland Security
Building 7, Chilmark
Salisbury, Wiltshire SP3 5DU, UK
Phone: +44 (0) 1722 717911
Fax: +44 (0) 1722 717941
eMail: [email protected]
Web: www.itseurope.co.uk

This manual contains valuable information about the nature of
ionizing radiation that should be understood by the user so that
accurate measurements can be made. Information on the care of
your Geiger counter is also included. If the following instructions
are followed, your radiation monitor will give you many years of
reliable service. For additional information please visit
www.sensafe.com/dx1.php.
These portable radiation detection devices are ideal for Homeland
Security and detecting environmental/industrial contamination
including the types released by nuclear power plants such as Iodine
and Cesium. The DX-1 and DX-2 instruments are very delicate
radiation detectors. Although housed in a high-impact case, the
Geiger-Müller (G-M) tube that senses radiation is fragile. If the unit
is dropped, the G-M tube may break. Exposure of the unit above
50° C (122° F) may also cause the G-M tube to stop functioning. The
electronic circuitry is sensitive to high humidity (over 90% R.H.).
CAUTION
DO NOT put the unit in a very hot place (such as a car's glove box
especially on a summer day).
DO NOT allow the unit to get wet. However, if this should happen,
clean it with a towel and allow unit to dry out for several
days in a dry place (not an oven).
DO NOT open the unit (except for battery replacement).There are no
adjustments inside for the DX-1 that can be made by the
user, since the unit is calibrated at the factory with special
equipment. For the DX-2, see instructions on page 5.
Battery Replacement
The unit is powered by a common 9-volt battery, and any 9-volt
battery will work. With the on-button activated, the LED should be
brightly lit. When an LED is no longer bright or when the LED dims
in the presence of a radiation source, replace battery. To replace the
battery:
1. Slide the plastic door 011 the unit located in the back.
2. Carefully replace the battery.
DO NOT put fingers into the unit through the battery compartment
while unit is on (G-M tube activation voltage is over 200 VDC).
3. Replace plastic door.
2

Operation
The radiation monitor only operates while the push button on the
face of the unit is depressed. This feature makes operation very
simple and conserves battery power. The unit is designed to be
held in the right hand with the thumb over the push button (see
Figures 1 and 2). The LED just above the push button indicates
that the unit is on and will give an indication of battery condition.
When the LED becomes dim or does not light, a new battery is
required.
When the unit is turned on, a faint buzz may be audible in a quiet
room. This is normal and is caused by the transformer that powers
the G-M tube.
In most parts of the world, background radiation will cause the
speaker to click at random intervals, about one click per every few
seconds. In areas where large deposits of natural radioactive
minerals are found, or in an area that has been contaminated with
radioactive materials, the speaker will click more frequently. This is
called the “background level.” It should be taken into account
when making measurements on specific objects.
Since the incidence of clicks from radioactive sources is random,
several clicks will be heard in rapid succession, while on other
occasions several seconds may elapse between clicks. This is
normal. Averaged over a period of time, the click rate should
remain relatively constant.
The Gold Standard Geiger-Müller tube is located behind the slots
in the upper edge of the case. The surface of the tube is very thin
(3mm). This allows beta radiation to penetrate and to be detected
with greater efficiency. (Beta rays and other types of radiation will
be discussed in the next section). This thin surface is fragile and
poking sharp objects through the slots could break the G-M tube.
Your Geiger counter is designed to be sensitive to:
1. Gamma radiation (Which includes X-rays).
2. Beta radiation.
3

Gamma radiation and X-rays can penetrate the plastic case with
comparative ease.
Beta radiation can most efficiently enter the case through the open
slots. Although Beta radiation is easily detected, it is difficult to
measure accurately. Therefore, when a radioactive object (such as
a “Fiesta” ceramic cup) is being searched for Beta radiation, the
open slots in the case should be positioned in such a way that they
are exposed to the object (see Figure 1).
If the unit shows a significantly higher click rate in this position,
you can be reasonably certain the object is giving off Beta
radiation.
Now position the unit as shown in Figure 2. In this position, where
radiation cannot pass directly through the slots (Beta radiation
travels in straight lines for the most part) only gamma and X-ray
radiation from the object will be detected. THIS IS THE POSITION
IN WHICH TO HOLD THE GEIGER COUNTER TO TAKE READINGS.
It is important to understand this, for high meter readings will
result from allowing Beta rays to be measured with the gamma
rays. The meter scale is calibrated by using gamma radiation only.
4

Readings
All units are calibrated at the factory using gamma radiation. The
radioactive gamma source used in the factory is Cesium-137 that
has been Beta shielded with .062" of Aluminum, and measuring
other radioisotopes (as would usually be the case) introduces
some amount of error. The error caused by this is usually very little.
Note that in the case of X-rays, the unit is very sensitive and
subsequently meter readings should be divided by about 5.
The DX-2 instruments have been calibrated in compliance with
NRC (US Nuclear Regulatory Commission) rules and regulations,
title 10, Chap. 1, Code of Federal Regulations, Part 34.25. For how
often you have to calibrate your unit, check with your local NRC.
However, recalibration is recommended after each repair or
change of the G-M tube. Since the DX-2 radiation monitor has an
oscillator, it can have minor adjustments/calibration by turning the
screw on the oscillator with a small screwdriver in the desired
direction: turning clockwise to decrease reading can be done at a
licensed place by a professional. The adjustment pot is readily
seen on the back of the DX-2.
In addition to the analog display, a special logarithmic scale has
been designed into the DX-1/DX-2. At radiation levels that are in
excess of the analog scale, the DX-1 will emit beeps at a rate that
increases as the radiation level increases above 10mR/hr.
Although this range is not as accurate as the meter, beeping will
begin approximately at 10mR/hr. A continuous beep occurs
approximately at 20mR/hr. These features greatly simplify
operation and allow reasonably quick and reasonably accurate
measurements to be made of radiation.
The analog display is not intended to indicate levels below
0.1mR/hr. However, very low level measurements can be made by
simply counting the clicks over a period of time much like taking a
person's pulse, and expressing the result as clicks (or counts) per
minute. 0.1 mR/hr on the meter corresponds to approximately 200
counts per minute (based on gamma emitting isotope).
5

Interpreting Readings
Health physics, the field that pertains to radiation and its effects on
man, is very complex, and theories and conclusions are constantly
being updated as information becomes available. Data from
occupational exposure, animal studies, and events like Hiroshima
and Nagasaki have fairly well established the maximum safe
exposure limits for man. Whether low level radiation causes cancer
and birth defects is still being debated. Delayed effect, which
could take years to develop, is difficult to study, and therefore,
there are no well-defined lower limits on ionizing radiation. Two
publications entitled “Hormesis with Ionizing Radiation,” 1980 and
“Radiation Hormesis,” 1991 (CRC Press, Boca Raton) present over
one thousand examples of statistically valid data showing no
physiological harm in vertebrates from the whole body exposures
to low dose radiation (<60mR per year).
As previously mentioned in the section on operation, the units
mR/hr (milli-Rem per hour, or 1/1000th of a Roentgen per hour)
pertain only to gamma radiation. Often other units of measurement
similar to mR/hr are used. The term REM (Roentgen Equivalent
Man) includes the affects of beta, alpha and neutron radiation.
Measurement in REMS is more complete as radiation affects man,
but such measurements are a complicated combination of many
measurements each made with specialized detector.
It is important to note that the field intensity from a radioactive
object decreases very qulckly with distance. If the object is very
small, increasing the distance from the object by a factor of two
decreases the radiation level by a factor of four. This is called a
square law situation, which demonstrates the dependence of
proximity on dose for small radioactive sources. Larger sources,
such as a large deposit of radioactive minerals, will show much
less of this effect. In trying to estimate the danger of radioactive
materials, it is important to take into account many aspects of the
situation. For instance, the radiation level at the face of a
radium-dial watch (common in the 1930's) may be 3mR/hr, but the
measurement taken from the back of the watch may be 0.3mR/hr.
6

Another interesting point concerns the energy of the radiation.
Geiger counters will register one click whenever they detect a ray
or particle of radiation hitting the G-M tube. These tiny high speed
bundles of energy are like short bursts of light. Some are extremely
energetic, while others are not. Geiger counters cannot determine
the energy of the impinging ray, they only detect its presence.
X-rays will be detected, but their energy is rather low.
Correspondingly, the meter readings should be divided by about 5
as compensation.
The opposite is true for cosmic rays (easily detected by DX-1/DX-2
in an airplane above 5,000 feet) which have enormous energy —
some millions of times more energetic than anything found here on
earth. The compensation figure for radiation of this type is difficult
to estimate, due to the extreme range of cosmic ray energies.
Radiation — What is it?
Nuclear physics is a very complex field, however, the basic
principle can be simply explained.
All matter is composed of atoms. Atoms alone and bonded
together in molecules form all the things around us, including
ourselves. These atomic units are extremely small; so small, in
fact, that a singe grain of table salt contains approximately
1,000,000,000,000,000,000 atoms (this is not a misprint). It is
impossible to see an atom, except with a sophisticated electron
microscope, so many of our present day theories on the structure
and composition of single atoms are based largely on the study of
radiation given off from unstable (radioactive) substances.
Atoms are composed of three basic particles: protons, neutrons and
electrons. Electrons are extremely light, negatively charged
particles that exist as a cloud around the center, or nucleus, of the
atom. Sometimes the electrons are said to occupy orbits around the
nucleus. These electrons are attracted to the nucleus because of
7

the positively charged protons that, along with the neutrons, make
up the nucleus. Atoms bond together in molecules when one atom
gives up or shares an electron with another atom. Chemical
reactions utilize this bonding process.
In all atoms, the number of electrons (and therefore the number of
negative charges) equals the number of protons (positive
charges). The number of protons or electrons in an atom
determines the chemical nature of the atom, and each element has
its own unique number (example: hydrogen = 1, helium = 2 etc.).
The number of neutrons, however, may not always be the same in
every atom of a particular element. Atoms of an element with
different numbers of neutrons are called isotopes. Every atom of a
particular element has the same atomic number, but different
isotopes of a given element have different atomic weights.
It is the variable number of neutrons in the nucleus of an atom that
leads to a process called nuclear decay that causes radiation.
When an atom has too many or too few neutrons in its nucleus it
will have a tendency to rearrange itself spontaneously into a new
combination of particles that are more stable. In this decay
process, bundles of excess energy are shot out of the nucleus in a
number of ways. Examples include:
1. When the neutrons are excessive, a neutron can convert itself to
a proton and shoot out an electron at very high speed, known as
beta radiation.
2. A proton may be converted to a neutron to cause an unusual
particle called a positron to be ejected from the nucleus.
3. In still another process, the nucleus, in a vain attempt to stabilize
itself, kicks out two protons and two neutrons all together as one
particle, called an alpha particle.
8

The energy released in each decay can be enormous. This decay
process is utilized in atomic reactors and bombs. When certain
very heavy isotopes of uranium or plutonium (or several other
isotopes) decay, they may split apart. This process is called
fission. In fission, the entire nucleus splits apart, causing two new
atoms and releasing a very large amount of energy. This process is
not very predictable, for the nucleus can split in many ways,
yielding a wide variety of new atoms and even some free neutrons.
The free neutrons, when released, can be absorbed by other fuel
atoms, causing them, in turn, to fission -- leading to a continuous
or (if not controlled) explosive chain reaction. Due to the wide
range of new atoms produced in the fission process, many of the
daughter products are not stable and will, in turn, decay
themselves, leading to hazardous nuclear waste and fallout.
In all of the above processes, another kind of radiation, gamma, is
almost always released. Unlike the particles previously mentioned,
gamma radiation consists of tiny discrete bundles of energy called
quanta. Light, X-rays and gamma rays can all be described as
quanta, the difference being the total energy packed into each
bundle.
In nuclear decay some energy in the unstable nucleus is dissipated
to its surroundings in the form of heat and radiation in the instant
that it decays. The nucleus may remain in its unstable state for
billions of years, and then suddenly decay spontaneously. The
time required for half of the atoms of a particular isotope to decay
is called the half-life of that isotope. For an isotope with a half-life
of 1 year, the pure isotope substance would be only 50% pure after
one year, half of the original atoms having decayed into some
other substance. After another year, 25% of the original material
would remain, and so on. Natural radioactive materials In our world
are only those with very, very long half-lives. Uranium-238, for
example, has a half-life of 4 1/2 billion years, and exists today only
because not enough time has elapsed since its creation for it to
decay away to negligible levels.
9

Scientists believe that the universe was created from a huge mass
of sub-atomic particles and energy — the Big Bang Theory.
Of the elements and their isotopes that constitute our planet, the
vast majority are quite stable, the result of billions of years of
nuclear decay. The amount of radiation given off from natural
radioactive minerals in the earth's crust is a major constituent of
background radiation. For the most part, its quite low, due to the
long time required for the remaining radioisotopes to decay. In
atomic reactions (either natural or forced by man) the decay
process is sped up by the effect of neutrons given off in the fission
process interacting with more unstable isotopes to cause
immediate decay. While this allows the energy of the isotope to be
harvested in a conveniently short time, the unstable decay
products produced generally have short half-lives, on the order of
seconds to centuries, and are very radioactive. As a result of this
process, considerable larger quantities of short half-live (high
decay rate) isotopes, such as Iodine-125, Iodine-131, and
Cesium-137, become a part of the world we live in. This is the
basis for the controversy and concerns on the subject of nuclear
power generation, waste disposal, and nuclear weapons.
Interaction of Radiation with Matter
The particles and photons that result from nuclear decay carry
most of the energy released from the original unstable nucleus.
The value of this energy is expressed in electron Volts, or eV. The
energy of beta and alpha rays is invested in the particles' speed. A
typical beta particle from Cesium-137 has an energy of about
500,000 eV, and a speed that approaches that of light. Beta
energies can cover a wide range, and many radioisotopes are
known to emit betas at energies in excess of 10 million eV. The
penetration range of typical beta particles is only a few millimeters
in human skin.
Alpha particles have even shorter penetration ranges than beta
particles. Typical alpha energies are on the order of 5 million eV,
with ranges so short that they are ext remely difficult to measure.
Alphas are stopped by a thin sheet of paper, and in air only travel
10

a few inches at most before coming to a stop. Therefore, alpha
particles cannot be detected without being in close contact with
the source, and even then only the alphas coming from the surface
of the source can be detected. Alphas generated within the source
are absorbed before reaching the surface. Due to short range,
alpha particles are not a serious health hazard unless they are
emitted from within the body when their high energy, in close
contact with sensitive living tissue, is an extreme hazard.
Fortunately, almost all alpha-emitting substances also emit
gamma rays, allowing for their detection.
Neutrons, having no net charge, do not interact with matter as
easily as other particles, and can drift through great thicknesses of
material without incident. A free neutron, drifting through space,
will decay in an average of 11.7 minutes, yielding a proton and an
electron (beta ray). The neutron can also combine with the nucleus
of an atom, if its path carries it close enough. When a neutron is
absorbed into a nucleus, it is saved from its ultimate fate (decay),
but may render the nucleus unstable. This absorption process is
used in medicine and industry, to create radioactive elements from
non-radioactive ones. Detecting neutrons is specialized and
beyond the scope of typical Geiger counters, but most possible
neutron sources also emit gamma and beta radiation, affording
detection of the source.
The highly energetic X-ray and gamma rays lose their energy as
they penetrate matter. X-rays have an energy of up to about
200,000 eV, compared to gamma radiation which can be as
energetic as several million eV. One million eV gamma radiation
can penetrate an inch of steel. Gamma and X-ray radiation are by
far the most penetrating of all common types, and are only
effectively absorbed by large amounts of heavy, dense material of
high atomic number, such as lead.
For other radiation basics, go to:
http://www.hps.org/publicinformation/ate/faqs/radiation.html
©2011 Industrial Test Systems, Inc. Rock Hill, SC Rev. 03/24/11
11

Limited Warranty
The DX-1 and the DX-2 Geiger counters are warrantied for 5 years
on electronics and 1 year for G-M tube from the date of purchase.
If a unit fails to function properly within the warranty period, the
manufacturer will repair or replace that unit, at its option. This
warranty does not cover any damage to the unit as a result of
misuse, accident or repair by unauthorized personnel.
Manufacturer reserves the right to make such determination on the
basis of factory inspection. All products returned for service must
be shipped prepaid. Call (803) 329-9712 for details.
Repair Charges (subject to change)
Replacement of G-M tube......................................................$80.00
Replacement of circuit board...............................................$120.00
(At time of repair, unit is factory recalibrated at no additional charge.)
Notice
Manufacturer believes Geiger counter to be accurate within
reasonable standards of acceptance, and includes instructions
that, if followed, will yield accurate measurements. Manufacturer
assumes no liability for damages. consequential or otherwise that
may arise for the use of the Geiger counter by any person, under
any circumstances. This Geiger counter is sensitive to gamma,
beta and X-ray radiation, but not necessarily to extremely low
energy forms, or alpha, neutron or microwave radiation. Do not
open Geiger counter, tamper with, or attempt to service it. Return
to manufacturer for all service repairs.
Instrument Specifications
DX-1 DX-2
Dual Scale 0-100μSv/Hr 01000μS/Hr
(Logarithmic) 0-10mR/Hr 0-100mR/Hr
Typical Accuracy ± 20% ± 15%
Temperature Range -10° to 40°C -10° to 40°C
G-M Tube Thickness 3mm 3mm
12
This manual suits for next models
1
Other Industrial Test Systems Measuring Instrument manuals
Popular Measuring Instrument manuals by other brands
Digipressure
Digipressure DPC_ HL-DIF Series Setting manual

YOKOGAWA
YOKOGAWA FLXA402T user manual

Maxwell Digital Multimeters
Maxwell Digital Multimeters MX-25403B user manual

Southwire
Southwire 41170S operating instructions

NexSens Technology
NexSens Technology WQ-DO user manual

Kobold
Kobold NPF operating instructions

Virtual Console
Virtual Console T1/E1 user manual

Graetz
Graetz GammaTwin Operation manual

CS Instruments
CS Instruments DS 500 Modbus Installation and operating instructions

Seitron
Seitron CHEMIST 600 BE GREEN Use and maintenance

GHM
GHM Delta OHM PYRAsense LPS03 Series operating manual

Fluke
Fluke i430-Flexi-TF Instruction