Electrostimulator «АВР-051» exercises effect, first of all, on vascular
tone. It is the most effective and safe way to correct blood pressure. In
this case, the device has practically no effect on cardiac output volume
or heart rate.
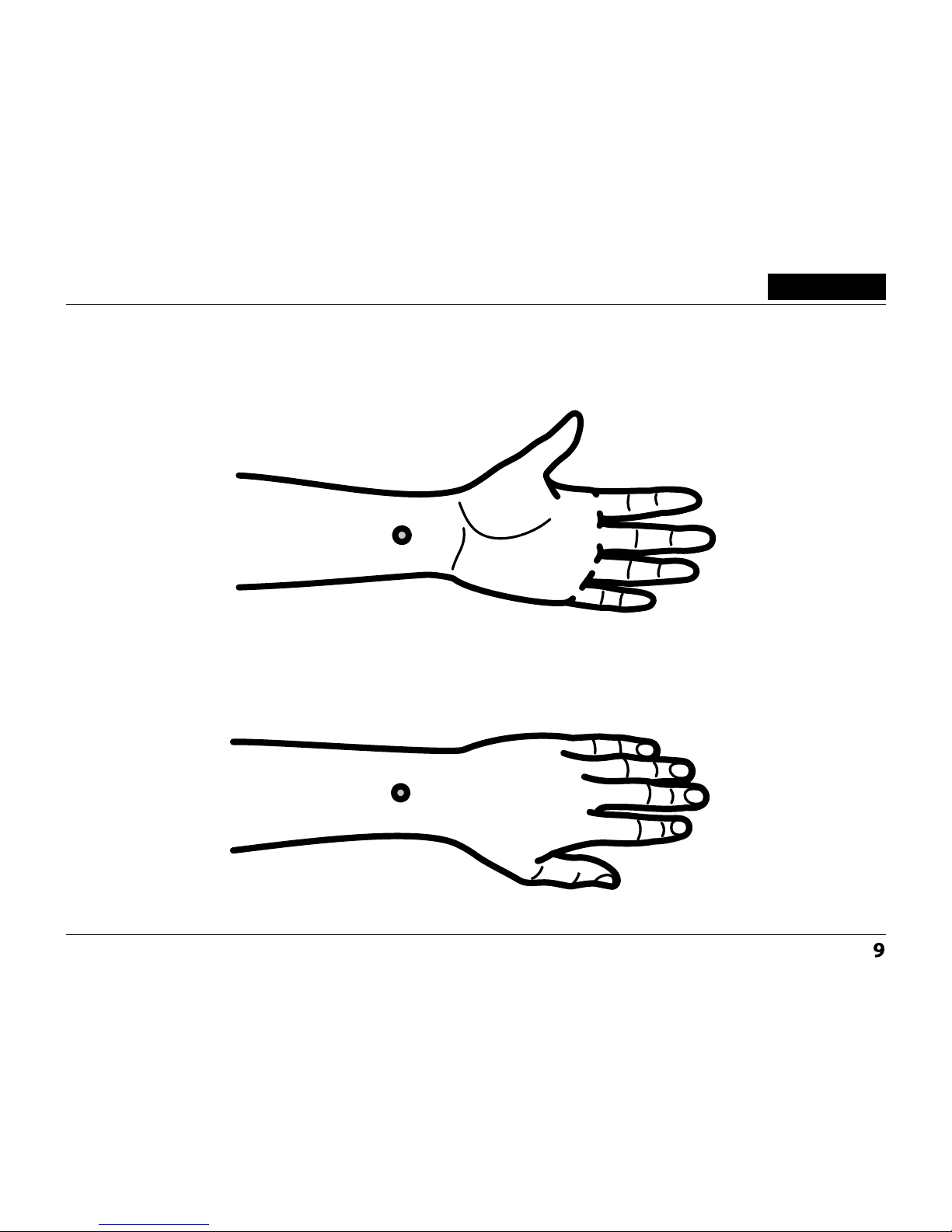
The choice of zones was determined by the type of disorder and
usability of device at work, at home, during the course of treatment.
Stimulation is effected with a group of impulses. The number of
groups corresponds with the frequencies. The treatment efficiency
depends on the patient’s state before the device usage.
Adaptation to electrostimulation develops less frequently and more
slowly, in addition, treatment with Electrostimulator «АВР-051» has
small intensity and duration; thus, portability and procedures safety
increase.
Electrostimulator is a mobile, light-weight and compact device that
allows you to carry out procedures at any convenient time, anywhere, as
well as:
• acts without subcutaneous introduction, has no risk of infection;
• is painless;
• period of zones treating depends on the selected mode;
• system is designed to be used with one hand in order to facilitate the
operation;
• no hospitalization required for the course of procedures.
English